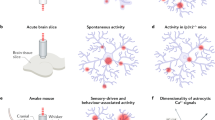
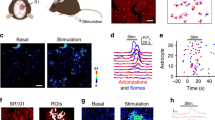
Abstract
The discovery that transient elevations of calcium concentration occur in astrocytes, and release 'gliotransmitters' which act on neurons and vascular smooth muscle, led to the idea that astrocytes are powerful regulators of neuronal spiking, synaptic plasticity and brain blood flow. These findings were challenged by a second wave of reports that astrocyte calcium transients did not mediate functions attributed to gliotransmitters and were too slow to generate blood flow increases. Remarkably, the tide has now turned again: the most important calcium transients occur in fine astrocyte processes not resolved in earlier studies, and new mechanisms have been discovered by which astrocyte [Ca2+]i is raised and exerts its effects. Here we review how this third wave of discoveries has changed our understanding of astrocyte calcium signaling and its consequences for neuronal function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bezzi, P. et al. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat. Neurosci. 7, 613–620 (2004).
Barres, B.A. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60, 430–440 (2008).
Fiacco, T.A., Agulhon, C. & McCarthy, K.D. Sorting out astrocyte physiology from pharmacology. Annu. Rev. Pharmacol. Toxicol. 49, 151–174 (2009).
Cornell-Bell, A.H., Finkbeiner, S.M., Cooper, M.S. & Smith, S.J. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science 247, 470–473 (1990).
Dani, J.W., Chernjavsky, A. & Smith, S.J. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron 8, 429–440 (1992).
Porter, J.T. & McCarthy, K.D. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J. Neurosci. 16, 5073–5081 (1996).
Newman, E.A. & Zahs, K.R. Calcium waves in retinal glial cells. Science 275, 844–847 (1997).
Wang, X. et al. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat. Neurosci. 9, 816–823 (2006).
Hirase, H., Qian, L., Barthó, P. & Buzsáki, G. Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol. 2, E96 (2004).
Nimmerjahn, A., Kirchhoff, F., Kerr, J.N. & Helmchen, F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat. Methods 1, 31–37 (2004).
Parpura, V. et al. Glutamate-mediated astrocyte-neuron signalling. Nature 369, 744–747 (1994).
Nedergaard, M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science 263, 1768–1771 (1994).
Pasti, L., Volterra, A., Pozzan, T. & Carmignoto, G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J. Neurosci. 17, 7817–7830 (1997).
Bezzi, P. et al. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature 391, 281–285 (1998).
Parri, H.R., Gould, T.M. & Crunelli, V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat. Neurosci. 4, 803–812 (2001).
Fellin, T. et al. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 43, 729–743 (2004).
Angulo, M.C., Kozlov, A.S., Charpak, S. & Audinat, E. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J. Neurosci. 24, 6920–6927 (2004).
Kang, J., Jiang, L., Goldman, S.A. & Nedergaard, M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat. Neurosci. 1, 683–692 (1998).
Newman, E.A. Propagation of intercellular calcium waves in retinal astrocytes and Müller cells. J. Neurosci. 21, 2215–2223 (2001).
Newman, E.A. Glial cell inhibition of neurons by release of ATP. J. Neurosci. 23, 1659–1666 (2003).
Pryazhnikov, E. & Khiroug, L. Sub-micromolar increase in [Ca2+]i triggers delayed exocytosis of ATP in cultured astrocytes. Glia 56, 38–49 (2008).
Yang, Y. et al. Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc. Natl. Acad. Sci. USA 100, 15194–15199 (2003).
Mothet, J.-P. et al. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc. Natl. Acad. Sci. USA 102, 5606–5611 (2005).
Henneberger, C., Papouin, T., Oliet, S.H. & Rusakov, D.A. Long-term potentiation depends on release of D-serine from astrocytes. Nature 463, 232–236 (2010).
Liu, Q.Y., Schaffner, A.E., Chang, Y.H., Maric, D. & Barker, J.L. Persistent activation of GABAA receptor/Cl− channels by astrocyte-derived GABA in cultured embryonic rat hippocampal neurons. J. Neurophysiol. 84, 1392–1403 (2000).
Kozlov, A.S., Angulo, M.C., Audinat, E. & Charpak, S. Target cell-specific modulation of neuronal activity by astrocytes. Proc. Natl. Acad. Sci. USA 103, 10058–10063 (2006).
Lee, S. et al. Channel-mediated tonic GABA release from glia. Science 330, 790–796 (2010).
Jiménez-González, C., Pirttimaki, T., Cope, D.W. & Parri, H.R. Non-neuronal, slow GABA signalling in the ventrobasal thalamus targets δ-subunit-containing GABA(A) receptors. Eur. J. Neurosci. 33, 1471–1482 (2011).
Beattie, E.C. et al. Control of synaptic strength by glial TNFα. Science 295, 2282–2285 (2002).
Allen, N.J. et al. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature 486, 410–414 (2012).
Diniz, L.P. et al. Astrocyte transforming growth factor beta 1 promotes inhibitory synapse formation via CaM kinase II signaling. Glia 62, 1917–1931 (2014).
Hamilton, N.B. & Attwell, D. Do astrocytes really exocytose neurotransmitters? Nat. Rev. Neurosci. 11, 227–238 (2010).
Perea, G. & Araque, A. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J. Neurosci. 25, 2192–2203 (2005).
D'Ascenzo, M. et al. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc. Natl. Acad. Sci. USA 104, 1995–2000 (2007).
Araque, A., Sanzgiri, R.P., Parpura, V. & Haydon, P.G. Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents in cultured hippocampal neurons. J. Neurosci. 18, 6822–6829 (1998).
Fiacco, T.A. & McCarthy, K.D. Intracellular astrocyte calcium waves in situ increase the frequency of spontaneous AMPA receptor currents in CA1 pyramidal neurons. J. Neurosci. 24, 722–732 (2004).
Jourdain, P. et al. Glutamate exocytosis from astrocytes controls synaptic strength. Nat. Neurosci. 10, 331–339 (2007).
Perea, G. & Araque, A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science 317, 1083–1086 (2007).
Pascual, O. et al. Astrocytic purinergic signaling coordinates synaptic networks. Science 310, 113–116 (2005).
Zhang, J.M. et al. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron 40, 971–982 (2003).
Serrano, A., Haddjeri, N., Lacaille, J.-C. & Robitaille, R. GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J. Neurosci. 26, 5370–5382 (2006).
Halassa, M.M. et al. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 61, 213–219 (2009).
Zonta, M. et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat. Neurosci. 6, 43–50 (2003).
Mulligan, S.J. & MacVicar, B.A. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 431, 195–199 (2004).
Gordon, G.R., Choi, H.B., Rungta, R.L., Ellis-Davies, G.C. & MacVicar, B.A. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 456, 745–749 (2008).
Attwell, D. et al. Glial and neuronal control of brain blood flow. Nature 468, 232–243 (2010).
Harris, J.J., Jolivet, R. & Attwell, D. Synaptic energy use and supply. Neuron 75, 762–777 (2012).
Loaiza, A., Porras, O.H. & Barros, L.F. Glutamate triggers rapid glucose transport stimulation in astrocytes as evidenced by real-time confocal microscopy. J. Neurosci. 23, 7337–7342 (2003).
Gourine, A.V. et al. Astrocytes control breathing through pH-dependent release of ATP. Science 329, 571–575 (2010).
Oberheim, N.A. et al. Uniquely hominid features of adult human astrocytes. J. Neurosci. 29, 3276–3287 (2009).
Han, X. et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 12, 342–353 (2013).
Carmignoto, G. & Haydon, P.G. Astrocyte calcium signaling and epilepsy. Glia 60, 1227–1233 (2012).
Hamby, M.E. et al. Inflammatory mediators alter the astrocyte transcriptome and calcium signaling elicited by multiple G-protein-coupled receptors. J. Neurosci. 32, 14489–14510 (2012).
Rossi, D. et al. Defective tumor necrosis factor-alpha-dependent control of astrocyte glutamate release in a transgenic mouse model of Alzheimer disease. J. Biol. Chem. 280, 42088–42096 (2005).
Lee, W. et al. Enhanced Ca2+-dependent glutamate release from astrocytes of the BACHD Huntington's disease mouse model. Neurobiol. Dis. 58, 192–199 (2013).
Bezzi, P. et al. CXCR4-activated astrocyte glutamate release via TNFα: amplification by microglia triggers neurotoxicity. Nat. Neurosci. 4, 702–710 (2001).
Takano, T. et al. Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 9, 260–267 (2006).
Cahoy, J.D. et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 28, 264–278 (2008).
Sun, W. et al. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science 339, 197–200 (2013).
Cai, Z., Schools, G.P. & Kimelberg, H.K. Metabotropic glutamate receptors in acutely isolated hippocampal astrocytes: developmental changes of mGluR5 mRNA and functional expression. Glia 29, 70–80 (2000).
Lavialle, M. et al. Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc. Natl. Acad. Sci. USA 108, 12915–12919 (2011).
Patrushev, I., Gavrilov, N., Turlapov, V. & Semyanov, A. Subcellular location of astrocytic calcium stores favors extrasynaptic neuron-astrocyte communication. Cell Calcium 54, 343–349 (2013).
Sahlender, D.A., Savtchouk, I. & Volterra, A. What do we know about gliotransmitter release from astrocytes? Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20130592 (2014).
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 (2014).
Fiacco, T.A. et al. Selective stimulation of astrocyte calcium in situ does not affect neuronal excitatory synaptic activity. Neuron 54, 611–626 (2007).
Petravicz, J., Fiacco, T.A. & McCarthy, K.D. Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J. Neurosci. 28, 4967–4973 (2008).
Agulhon, C., Fiacco, T.A. & McCarthy, K.D. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science 327, 1250–1254 (2010).
Navarrete, M. et al. Astrocytes mediate in vivo cholinergic-induced synaptic plasticity. PLoS Biol. 10, e1001259 (2012).
Takata, N. et al. Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J. Neurosci. 31, 18155–18165 (2011).
Bonder, D.E. & McCarthy, K.D. Astrocytic Gq-GPCR-linked IP3R-dependent Ca2+ signaling does not mediate neurovascular coupling in mouse visual cortex in vivo. J. Neurosci. 34, 13139–13150 (2014).
Nizar, K. et al. In vivo stimulus-induced vasodilation occurs without IP3 receptor activation and may precede astrocytic calcium increase. J. Neurosci. 33, 8411–8422 (2013).
Petravicz, J., Boyt, K.M. & McCarthy, K.D. Astrocyte IP3R2-dependent Ca2+ signaling is not a major modulator of neuronal pathways governing behavior. Front. Behav. Neurosci. 8, 384 (2014).
Wang, F. et al. Photolysis of caged Ca2+ but not receptor-mediated Ca2+ signaling triggers astrocytic glutamate release. J. Neurosci. 33, 17404–17412 (2013).
Shigetomi, E., Bowser, D.N., Sofroniew, M.V. & Khakh, B.S. Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. J. Neurosci. 28, 6659–6663 (2008).
Schummers, J., Yu, H. & Sur, M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science 320, 1638–1643 (2008).
Schulz, K. et al. Simultaneous BOLD fMRI and fiber-optic calcium recording in rat neocortex. Nat. Methods 9, 597–602 (2012).
Winship, I.R., Plaa, N. & Murphy, T.H. Rapid astrocyte calcium signals correlate with neuronal activity and onset of the hemodynamic response in vivo. J. Neurosci. 27, 6268–6272 (2007).
Lalo, U. et al. Exocytosis of ATP from astrocytes modulates phasic and tonic inhibition in the neocortex. PLoS Biol. 12, e1001747 (2014).
Santello, M., Bezzi, P. & Volterra, A. TNFα controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron 69, 988–1001 (2011).
Takano, T. et al. Receptor-mediated glutamate release from volume sensitive channels in astrocytes. Proc. Natl. Acad. Sci. USA 102, 16466–16471 (2005).
Li, D. et al. Lack of evidence for vesicular glutamate transporter expression in mouse astrocytes. J. Neurosci. 33, 4434–4455 (2013).
Fujita, T. et al. Neuronal transgene expression in dominant-negative SNARE mice. J. Neurosci. 34, 16594–16604 (2014).
Sloan, S.A. & Barres, B.A. Looks can be deceiving: reconsidering the evidence for gliotransmission. Neuron 84, 1112–1115 (2014).
Sultan, S. et al. Synaptic integration of adult-born hippocampal neurons is locally controlled by astrocytes. Neuron 88, 1–16 (2015).
Su, M. et al. Expression specificity of GFAP transgenes. Neurochem. Res. 29, 2075–2093 (2004).
Araque, A., Li, N., Doyle, R.T. & Haydon, P.G. SNARE protein-dependent glutamate release from astrocytes. J. Neurosci. 20, 666–673 (2000).
Jurado, S. et al. LTP requires a unique postsynaptic SNARE fusion machinery. Neuron 77, 542–558 (2013).
Feldmann, A. et al. Transport of the major myelin proteolipid protein is directed by VAMP3 and VAMP7. J. Neurosci. 31, 5659–5672 (2011).
Moreau, K. et al. PICALM modulates autophagy activity and tau accumulation. Nat. Commun. 5, 4998 (2014).
Nett, W.J., Oloff, S.H. & McCarthy, K.D. Hippocampal astrocytes in situ exhibit calcium oscillations that occur independent of neuronal activity. J. Neurophysiol. 87, 528–537 (2002).
Grosche, J. et al. Microdomains for neuron-glia interaction: parallel fiber signaling to Bergmann glial cells. Nat. Neurosci. 2, 139–143 (1999).
Nimmerjahn, A., Mukamel, E.A. & Schnitzer, M.J. Motor behavior activates Bergmann glial networks. Neuron 62, 400–412 (2009).
Kanemaru, K. et al. In vivo visualization of subtle, transient, and local activity of astrocytes using an ultrasensitive Ca2+ indicator. Cell Reports 8, 311–318 (2014).
Shigetomi, E., Kracun, S., Sofroniew, M.V. & Khakh, B.S. A genetically targeted optical sensor to monitor calcium signals in astrocyte processes. Nat. Neurosci. 13, 759–766 (2010).
Shigetomi, E., Tong, X., Kwan, K.Y., Corey, D.P. & Khakh, B.S. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat. Neurosci. 15, 70–80 (2012).
Di Castro, M.A. et al. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat. Neurosci. 14, 1276–1284 (2011).
Panatier, A. et al. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 146, 785–798 (2011).
Chen, T.-W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Sibille, J., Zapata, J., Teillon, J. & Rouach, N. Astroglial calcium signaling displays short-term plasticity and adjusts synaptic efficacy. Front. Cell. Neurosci. 9, 189 (2015).
Srinivasan, R. et al. Ca2+ signaling in astrocytes from Ip3r2−/− mice in brain slices and during startle responses in vivo. Nat. Neurosci. 18, 708–717 (2015).
Tang, W. et al. Stimulation-evoked Ca2+ signals in astrocytic processes at hippocampal CA3-CA1 synapses of adult mice are modulated by glutamate and ATP. J. Neurosci. 35, 3016–3021 (2015).
Lind, B.L., Brazhe, A.R., Jessen, S.B., Tan, F.C.C. & Lauritzen, M.J. Rapid stimulus-evoked astrocyte Ca2+ elevations and hemodynamic responses in mouse somatosensory cortex in vivo. Proc. Natl. Acad. Sci. USA 110, E4678–E4687 (2013).
Otsu, Y. et al. Calcium dynamics in astrocyte processes during neurovascular coupling. Nat. Neurosci. 18, 210–218 (2015).
Hamilton, N. et al. Mechanisms of ATP- and glutamate-mediated calcium signaling in white matter astrocytes. Glia 56, 734–749 (2008).
Palygin, O., Lalo, U., Verkhratsky, A. & Pankratov, Y. Ionotropic NMDA and P2X1/5 receptors mediate synaptically induced Ca2+ signalling in cortical astrocytes. Cell Calcium 48, 225–231 (2010).
Newman, E.A. Calcium increases in retinal glial cells evoked by light-induced neuronal activity. J. Neurosci. 25, 5502–5510 (2005).
Pankratov, Y. & Lalo, U. Role for astroglial α1-adrenoreceptors in gliotransmission and control of synaptic plasticity in the neocortex. Front. Cell. Neurosci. 9, 230 (2015).
Bowser, D.N. & Khakh, B.S. Vesicular ATP is the predominant cause of intercellular calcium waves in astrocytes. J. Gen. Physiol. 129, 485–491 (2007).
Haustein, M.D. et al. Conditions and constraints for astrocyte calcium signaling in the hippocampal mossy fiber pathway. Neuron 82, 413–429 (2014).
Doengi, M. et al. GABA uptake-dependent Ca2+ signaling in developing olfactory bulb astrocytes. Proc. Natl. Acad. Sci. USA 106, 17570–17575 (2009).
Duffy, S. & MacVicar, B.A. Adrenergic calcium signaling in astrocyte networks within the hippocampal slice. J. Neurosci. 15, 5535–5550 (1995).
Shelton, M.K. & McCarthy, K.D. Hippocampal astrocytes exhibit Ca2+-elevating muscarinic cholinergic and histaminergic receptors in situ. J. Neurochem. 74, 555–563 (2000).
Paukert, M. et al. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 82, 1263–1270 (2014).
Ding, F. α1-Adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium 54, 387–394 (2013).
Chen, N. et al. Nucleus basalis-enabled stimulus-specific plasticity in the visual cortex is mediated by astrocytes. Proc. Natl. Acad. Sci. USA 109, E2832–E2841 (2012).
Thrane, A.S. et al. General anesthesia selectively disrupts astrocyte calcium signaling in the awake mouse cortex. Proc. Natl. Acad. Sci. USA 109, 18974–18979 (2012).
Woo, D.H. et al. TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell 151, 25–40 (2012).
Park, H. et al. High glutamate permeability and distal localization of Best1 channel in CA1 hippocampal astrocyte. Mol. Brain 6, 54 (2013).
Beppu, K. et al. Optogenetic countering of glial acidosis suppresses glial glutamate release and ischemic brain damage. Neuron 81, 314–320 (2014).
Devaraju, P., Sun, M.Y., Myers, T.L., Lauderdale, K. & Fiacco, T.A. Astrocytic group I mGluR-dependent potentiation of astrocytic glutamate and potassium uptake. J. Neurophysiol. 109, 2404–2414 (2013).
Mashimo, M. et al. Inositol 1,4,5-trisphosphate signaling maintains the activity of glutamate uptake in Bergmann glia. Eur. J. Neurosci. 32, 1668–1677 (2010).
Kalandadze, A., Wu, Y. & Robinson, M.B. Protein kinase C activation decreases cell surface expression of the GLT-1 subtype of glutamate transporter. Requirement of a carboxyl-terminal domain and partial dependence on serine 486. J. Biol. Chem. 277, 45741–45750 (2002).
Murphy-Royal, C. et al. Surface diffusion of astrocytic glutamate transporters shapes synaptic transmission. Nat. Neurosci. 18, 219–226 (2015).
Iino, M. et al. Glia-synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science 292, 926–929 (2001).
Bernardinelli, Y. et al. Activity-dependent structural plasticity of perisynaptic astrocytic domains promotes excitatory synapse stability. Curr. Biol. 24, 1679–1688 (2014).
Wang, F. et al. Astrocytes modulate neural network activity by Ca2+-dependent uptake of extracellular K+. Sci. Signal. 5, ra26 (2012).
Volterra, A., Liaudet, N. & Savtchouk, I. Astrocyte Ca2+ signalling: an unexpected complexity. Nat. Rev. Neurosci. 15, 327–335 (2014).
Rusakov, D.A. Disentangling calcium-driven astrocyte physiology. Nat. Rev. Neurosci. 16, 226–233 (2015).
Nimmerjahn, A. & Bergles, D.E. Large-scale recording of astrocyte activity. Curr. Opin. Neurobiol. 32, 95–106 (2015).
Li, W., Llopis, J., Whitney, M., Zlokarnik, G. & Tsien, R.Y. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature 392, 936–941 (1998).
Min, R. & Nevian, T. Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nat. Neurosci. 15, 746–753 (2012).
Navarrete, M. & Araque, A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 68, 113–126 (2010).
Holmström, K.M. et al. Signalling properties of inorganic polyphosphate in the mammalian brain. Nat. Commun. 4, 1362 (2013).
Filosa, J.A. et al. Endothelin-mediated calcium responses in supraoptic nucleus astrocytes influence magnocellular neurosecretory firing activity. J. Neuroendocrinol. 24, 378–392 (2012).
Bernardinelli, Y. et al. Astrocytes display complex and localized calcium responses to single-neuron stimulation in the hippocampus. J. Neurosci. 31, 8905–8919 (2011).
Morquette, P. et al. An astrocyte-dependent mechanism for neuronal rhythmogenesis. Nat. Neurosci. 18, 844–854 (2015).
Wang, H.C. et al. Spontaneous activity of cochlear hair cells triggered by fluid secretion mechanism in adjacent support cells. Cell 163, 1348–1359 (2015).
Acknowledgements
We thank N. Hamilton, R. Jolivet, A. Krasnow and A. Mishra for comments on the manuscript. Supported by the Wellcome Trust and European Research Council.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bazargani, N., Attwell, D. Astrocyte calcium signaling: the third wave. Nat Neurosci 19, 182–189 (2016). https://doi.org/10.1038/nn.4201
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4201
This article is cited by
-
Cortical astrocyte N-methyl-D-aspartate receptors influence whisker barrel activity and sensory discrimination in mice
Nature Communications (2024)
-
Hypothalamic astrocyte NAD+ salvage pathway mediates the coupling of dietary fat overconsumption in a mouse model of obesity
Nature Communications (2024)
-
Localized calcium transients in phragmoplast regulate cytokinesis of tobacco BY-2 cells
Plant Cell Reports (2024)
-
Excitatory and inhibitory neuronal signaling in inflammatory and diabetic neuropathic pain
Molecular Medicine (2023)
-
Role of spinal astrocytes through the perisynaptic astrocytic process in pathological pain
Molecular Brain (2023)