Abstract
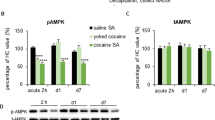
Using a rat model of craving and relapse, we have previously found time-dependent increases in cue-induced cocaine seeking over the first months of withdrawal from cocaine, suggesting that drug craving incubates over time. Here, we explored the role of the amygdala extracellular signal–regulated kinase (ERK) signaling pathway in this incubation. Cocaine seeking induced by exposure to cocaine cues was substantially higher after 30 withdrawal days than after 1 withdrawal day. Exposure to these cues increased ERK phosphorylation in the central, but not the basolateral, amygdala after 30 d, but not 1 d, of withdrawal. After 30 d of withdrawal from cocaine, inhibition of central, but not basolateral, amygdala ERK phosphorylation decreased cocaine seeking. After 1 d of withdrawal, stimulation of central amygdala ERK phosphorylation increased cocaine seeking. Results suggest that the incubation of cocaine craving is mediated by time-dependent increases in the responsiveness of the central amygdala ERK pathway to cocaine cues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
O'Brien, C.P. A range of research-based pharmacotherapies for addiction. Science 278, 66–70 (1997).
Gawin, F.H. & Kleber, H.D. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch. Gen. Psychiatry 43, 107–113 (1986).
Grimm, J.W., Hope, B.T., Wise, R.A. & Shaham, Y. Incubation of cocaine craving after withdrawal. Nature 412, 141–142 (2001).
Lu, L., Grimm, J.W., Dempsey, J. & Shaham, Y. Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology (Berl.) 176, 101–108 (2004).
Neisewander, J.L. et al. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J. Neurosci. 20, 798–805 (2000).
Nestler, E.J. Molecular basis of long-term plasticity underlying addiction. Nat. Rev. Neurosci. 2, 119–128 (2001).
Kalivas, P.W. Glutamate systems in cocaine addiction. Curr. Opin. Pharmacol. 4, 23–29 (2004).
Stewart, J. Pathways to relapse: factors controlling the reinitiation of drug seeking after abstinence. Nebr. Symp. Motiv. 50, 197–234 (2004).
Everitt, B.J. & Wolf, M.E. Psychomotor stimulant addiction: a neural systems perspective. J. Neurosci. 22, 3312–3320 (2002).
Grimm, J.W. et al. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J. Neurosci. 23, 742–747 (2003).
Lu, L., Grimm, J.W., Shaham, Y. & Hope, B.T. Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. J. Neurochem. 85, 1604–1613 (2003).
Lu, L., Dempsey, J., Liu, S.Y., Bossert, J.M. & Shaham, Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J. Neurosci. 24, 1604–1611 (2004).
Lu, L., Grimm, J.W., Hope, B.T. & Shaham, Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology 47 (Suppl. 1), 214–226 (2004).
Berhow, M.T., Hiroi, N. & Nestler, E.J. Regulation of ERK (extracellular signal regulated kinase), part of the neurotrophin signal transduction cascade, in the rat mesolimbic dopamine system by chronic exposure to morphine or cocaine. J. Neurosci. 16, 4707–4715 (1996).
Valjent, E. et al. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J. Neurosci. 20, 8701–8709 (2000).
Licata, S.C. & Pierce, R.C. The roles of calcium/calmodulin-dependent and Ras/mitogen-activated protein kinases in the development of psychostimulant-induced behavioral sensitization. J. Neurochem. 85, 14–22 (2003).
Thomas, G.M. & Huganir, R.L. MAPK cascade signalling and synaptic plasticity. Nat. Rev. Neurosci. 5, 173–183 (2004).
Adams, J.P. & Sweatt, J.D. Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu. Rev. Pharmacol. Toxicol. 42, 135–163 (2002).
Schafe, G.E. et al. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J. Neurosci. 20, 8177–8187 (2000).
Lu, K.T., Walker, D.L. & Davis, M. Mitogen-activated protein kinase cascade in the basolateral nucleus of amygdala is involved in extinction of fear-potentiated startle. J. Neurosci. 21, RC162 (2001).
LeDoux, J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 23, 155–184 (2000).
Gallagher, M. & Chiba, A.A. The amygdala and emotion. Curr. Opin. Neurobiol. 6, 221–227 (1996).
Everitt, B.J. et al. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann. NY Acad. Sci. 877, 412–438 (1999).
See, R.E. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol. Biochem. Behav. 71, 517–529 (2002).
Shaham, Y., Shalev, U., Lu, L., De Wit, H. & Stewart, J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl.) 168, 3–20 (2003).
Shalev, U., Grimm, J.W. & Shaham, Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol. Rev. 54, 1–42 (2002).
Balleine, B.W. & Dickinson, A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology 37, 407–419 (1998).
Davies, S.P., Reddy, H., Caivano, M. & Cohen, P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351, 95–105 (2000).
Fiore, R.S., Murphy, T.H., Sanghera, J.S., Pelech, S.L. & Baraban, J.M. Activation of p42 mitogen-activated protein kinase by glutamate receptor stimulation in rat primary cortical cultures. J. Neurochem. 61, 1626–1633 (1993).
Jentsch, J.D. & Taylor, J.R. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharamacology 146, 373–390 (1999).
Burns, L.H., Robbins, T.W. & Everitt, B.J. Differential effects of excitotoxic lesions of the basolateral amygdala, ventral subiculum and medial prefrontal cortex on responding with conditioned reinforcement and locomotor activity potentiated by intra-accumbens infusions of D-amphetamine. Behav. Brain Res. 55, 167–183 (1993).
Robledo, P., Robbins, T.W. & Everitt, B.J. Effects of excitotoxic lesions of the central amygdaloid nucleus on the potentiation of reward-related stimuli by intra-accumbens amphetamine. Behav. Neurosci. 110, 981–990 (1996).
Wolf, M.E., Sun, X., Mangiavacchi, S. & Chao, S.Z. Psychomotor stimulants and neuronal plasticity. Neuropharmacology 47 (Suppl. 1), 61–79 (2004).
Holland, P.C. & Gallagher, M. Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. Eur. J. Neurosci. 17, 1680–1694 (2003).
Ungless, M.A., Whistler, J.L., Malenka, R.C. & Bonci, A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411, 583–587 (2001).
Thomas, M.J., Beurrier, C., Bonci, A. & Malenka, R.C. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat. Neurosci. 4, 1217–1223 (2001).
Baker, D.A. et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat. Neurosci. 6, 743–749 (2003).
Wise, R.A. & Hoffman, D.C. Localization of drug reward mechanisms by intracranial injections. Synapse 10, 247–263 (1992).
Overton, D.A. Experimental methods for the study of state-dependent learning. Fed. Proc. 33, 1800–1813 (1974).
Whitelaw, R.B., Markou, A., Robbins, T.W. & Everitt, B.J. Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcement. Psychopharmacology (Berl.) 127, 213–224 (1996).
Davis, M., Walker, D.L. & Myers, K.M. Role of the amygdala in fear extinction measured with potentiated startle. Ann. NY Acad. Sci. 985, 218–232 (2003).
Berman, D.E. & Dudai, Y. Memory extinction, learning anew, and learning the new: dissociations in the molecular machinery of learning in cortex. Science 291, 2417–2419 (2001).
Nestler, E.J. Common molecular and cellular substrates of addiction and memory. Neurobiol. Learn. Mem. 78, 637–647 (2002).
Robinson, T.E. & Berridge, K.C. Addiction. Annu. Rev. Psychol. 54, 25–53 (2003).
Sweatt, J.D. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J. Neurochem. 76, 1–10 (2001).
Grant, S. et al. Activation of memory circuits during cue-elicited cocaine craving. Proc. Natl. Acad. Sci. USA 93, 12040–12045 (1996).
Eysenck, H.J. A theory of the incubation of anxiety-fear responses. Behav. Res. Ther. 6, 309–321 (1968).
Paxinos, G. & Watson, C. The Rat Brain in Stereotaxic Coordinates (Academic, San Diego, 1998).
Hayes, R.J., Vorel, S.R., Spector, J., Liu, X. & Gardner, E.L. Electrical and chemical stimulation of the basolateral complex of the amygdala reinstates cocaine-seeking behavior in the rat. Psychopharmacology (Berl.) 168, 75–83 (2003).
Kelleher, R.J. III, Govindarajan, A., Jung, H.Y., Kang, H. & Tonegawa, S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell 116, 467–479 (2004).
Acknowledgements
We thank S. Gray, D. Nagarkar, D. Chuang and C. Scheidweiler for technical assistance, and J. Stewart, D.E. Epstein, B.J. Everitt and R.A. Wise for helpful comments and critical discussions of the present data. Research was supported by the National Institute on Drug Abuse Intramural Research Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lu, L., Hope, B., Dempsey, J. et al. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci 8, 212–219 (2005). https://doi.org/10.1038/nn1383
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1383
This article is cited by
-
Memory consolidation drives the enhancement of remote cocaine memory via prefrontal circuit
Molecular Psychiatry (2024)
-
The anterior insula and its projection to amygdala nuclei modulate the abstinence-exacerbated expression of conditioned place preference
Psychopharmacology (2024)
-
Persistent increase of accumbens cocaine ensemble excitability induced by IRK downregulation after withdrawal mediates the incubation of cocaine craving
Molecular Psychiatry (2023)
-
Preventing incubation of drug craving to treat drug relapse: from bench to bedside
Molecular Psychiatry (2023)
-
Arc controls alcohol cue relapse by a central amygdala mechanism
Molecular Psychiatry (2023)