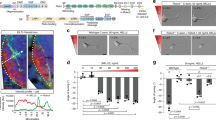
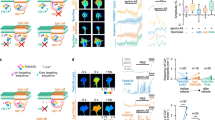
Abstract
Axon growth is governed by the ability of growth cones to interpret attractive and repulsive guidance cues. Recent studies have shown that secreted signaling molecules known as morphogens can also act as axon guidance cues. Of the large family of Wnt signaling components, only Wnt4 and Wnt5 seem to participate directly in axon guidance. Here we show that secreted Frizzled-related protein 1 (SFRP1), a proposed Wnt signaling inhibitor, can directly modify and reorient the growth of chick and Xenopus laevis retinal ganglion cell axons. This activity does not require Wnt inhibition and is modulated by extracellular matrix molecules. Intracellularly, SFRP1 function requires Gα protein activation, protein synthesis and degradation, and it is modulated by cyclic nucleotide levels. Because SFRP1 interacts with Frizzled-2 (Fz2) and interference with Fz2 expression abolishes growth cone responses to SFRP1, we propose a previously unknown function for this molecule: the ability to guide growth cone movement via the Fz2 receptor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ciani, L. & Salinas, P.C. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat. Rev. Neurosci. 6, 351–362 (2005).
Wang, H.Y. & Malbon, C.C. Wnt-frizzled signaling to G-protein-coupled effectors. Cell. Mol. Life Sci. 61, 69–75 (2004).
Packard, M. et al. The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell 111, 319–330 (2002).
Sanchez-Camacho, C., Rodriguez, J., Ruiz, J., Trousse, F. & Bovolenta, P. Morphogens as growth cone signalling molecules. Brain Res. Brain Res. Rev. 49, 242–252 (2005).
Yoshikawa, S., McKinnon, R.D., Kokel, M. & Thomas, J.B. Wnt-mediated axon guidance via the Drosophila Derailed receptor. Nature 422, 583–588 (2003).
Lyuksyutova, A.I. et al. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science 302, 1984–1988 (2003).
Wang, Y., Thekdi, N., Smallwood, P.M., Macke, J.P. & Nathans, J. Frizzled-3 is required for the development of major fiber tracts in the rostral CNS. J. Neurosci. 22, 8563–8573 (2002).
Lu, W., Yamamoto, V., Ortega, B. & Baltimore, D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell 119, 97–108 (2004).
Kawano, Y. & Kypta, R. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 116, 2627–2634 (2003).
Collavin, L. & Kirschner, M.W. The secreted Frizzled-related protein Sizzled functions as a negative feedback regulator of extreme ventral mesoderm. Development 130, 805–816 (2003).
Esteve, P., Trousse, F., Rodriguez, J. & Bovolenta, P. SFRP1 modulates retina cell differentiation through a beta-catenin-independent mechanism. J. Cell Sci. 116, 2471–2481 (2003).
Yabe, T. et al. Ogon/Secreted Frizzled functions as a negative feedback regulator of Bmp signaling. Development 130, 2705–2716 (2003).
Lee, J.L., Lin, C.T., Chueh, L.L. & Chang, C.J. Autocrine/paracrine secreted Frizzled-related protein 2 induces cellular resistance to apoptosis: a possible mechanism of mammary tumorigenesis. J. Biol. Chem. 279, 14602–14609 (2004).
Esteve, P., Lopez-Rios, J. & Bovolenta, P. SFRP1 is required for the proper establishment of the eye field in the medaka fish. Mech. Dev. 121, 687–701 (2004).
Esteve, P., Morcillo, J. & Bovolenta, P. Early and dynamic expression of cSfrp1 during chick embryo development. Mech. Dev. 97, 217–221 (2000).
Xu, Q., D'Amore, P.A. & Sokol, S.Y. Functional and biochemical interactions of Wnts with FrzA, a secreted Wnt antagonist. Development 125, 4767–4776 (1998).
Lohof, A.M., Quillan, M., Dan, Y. & Poo, M.M. Asymmetric modulation of cytosolic cAMP activity induces growth cone turning. J. Neurosci. 12, 1253–1261 (1992).
Hopker, V.H., Shewan, D., Tessier-Lavigne, M., Poo, M. & Holt, C. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature 401, 69–73 (1999).
Weinl, C., Drescher, U., Lang, S., Bonhoeffer, F. & Loschinger, J. On the turning of Xenopus retinal axons induced by ephrin-A5. Development 130, 1635–1643 (2003).
Hornberger, M.R. et al. Modulation of EphA receptor function by coexpressed ephrinA ligands on retinal ganglion cell axons. Neuron 22, 731–742 (1999).
Irie, A., Yates, E.A., Turnbull, J.E. & Holt, C.E. Specific heparan sulfate structures involved in retinal axon targeting. Development 129, 61–70 (2002).
Song, H. & Poo, M. The cell biology of neuronal navigation. Nat. Cell Biol. 3, E81–E88 (2001).
Nishiyama, M. et al. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature 423, 990–995 (2003).
Campbell, D.S. & Holt, C.E. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron 32, 1013–1026 (2001).
Chong, J.M., Uren, A., Rubin, J.S. & Speicher, D.W. Disulfide bond assignments of secreted Frizzled-related protein-1 provide insights about Frizzled homology and netrin modules. J. Biol. Chem. 277, 5134–5144 (2002).
Banyai, L. & Patthy, L. The NTR module: domains of netrins, secreted frizzled related proteins, and type I procollagen C-proteinase enhancer protein are homologous with tissue inhibitors of metalloproteases. Protein Sci. 8, 1636–1642 (1999).
Lim, Y.S. & Wadsworth, W.G. Identification of domains of netrin UNC-6 that mediate attractive and repulsive guidance and responses from cells and growth cones. J. Neurosci. 22, 7080–7087 (2002).
Willert, K. et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423, 448–452 (2003).
Uren, A. et al. Secreted frizzled-related protein-1 binds directly to Wingless and is a biphasic modulator of Wnt signaling. J. Biol. Chem. 275, 4374–4382 (2000).
Bafico, A. et al. Interaction of frizzled related protein (FRP) with Wnt ligands and the frizzled receptor suggests alternative mechanisms for FRP inhibition of Wnt signaling. J. Biol. Chem. 274, 16180–16187 (1999).
Dann, C.E. et al. Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature 412, 86–90 (2001).
Kuhl, M. The WNT/calcium pathway: biochemical mediators, tools and future requirements. Front. Biosci. 9, 967–974 (2004).
Chapman, S.C., Brown, R., Lees, L., Schoenwolf, G.C. & Lumsden, A. Expression analysis of chick Wnt and frizzled genes and selected inhibitors in early chick patterning. Dev. Dyn. 229, 668–676 (2004).
Xiang, Y. et al. Nerve growth cone guidance mediated by G protein-coupled receptors. Nat. Neurosci. 5, 843–848 (2002).
van Horck, F.P., Weinl, C. & Holt, C.E. Retinal axon guidance: novel mechanisms for steering. Curr. Opin. Neurobiol. 14, 61–66 (2004).
McLaughlin, T., Hindges, R. & O'Leary, D.D. Regulation of axial patterning of the retina and its topographic mapping in the brain. Curr. Opin. Neurobiol. 13, 57–69 (2003).
Morissette, N. & Carbonetto, S. Laminin alpha 2 chain (M chain) is found within the pathway of avian and murine retinal projections. J. Neurosci. 15, 8067–8082 (1995).
Deiner, M.S. et al. Netrin-1 and DCC mediate axon guidance locally at the optic disc: loss of function leads to optic nerve hypoplasia. Neuron 19, 575–589 (1997).
Deiner, M.S. & Sretavan, D.W. Altered midline axon pathways and ectopic neurons in the developing hypothalamus of netrin-1- and DCC-deficient mice. J. Neurosci. 19, 9900–9912 (1999).
Chuman, Y. et al. Identification of a peptide binding motif for secreted frizzled-related protein-1. Peptides 25, 1831–1838 (2004).
Slusarski, D.C., Corces, V.G. & Moon, R.T. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature 390, 410–413 (1997).
Ahumada, A. et al. Signaling of rat Frizzled-2 through phosphodiesterase and cyclic GMP. Science 298, 2006–2010 (2002).
Jin, E.J., Burrus, L.W. & Erickson, C.A. The expression patterns of Wnts and their antagonists during avian eye development. Mech. Dev. 116, 173–176 (2002).
Liu, H., Mohamed, O., Dufort, D. & Wallace, V.A. Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev. Dyn. 227, 323–334 (2003).
Kubo, F., Takeichi, M. & Nakagawa, S. Wnt2b controls retinal cell differentiation at the ciliary marginal zone. Development 130, 587–598 (2003).
Xu, Q. et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 116, 883–895 (2004).
Suzuki, H. et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat. Genet. 36, 417–422 (2004).
Topol, L. et al. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J. Cell Biol. 162, 899–908 (2003).
Kanekar, S. et al. Xath5 participates in a network of bHLH genes in the developing Xenopus retina. Neuron 19, 981–994 (1997).
Trousse, F., Marti, E., Gruss, P., Torres, M. & Bovolenta, P. Control of retinal ganglion cell axon growth: a new role for Sonic hedgehog. Development 128, 3927–3936 (2001).
Acknowledgements
We sincerely thank M. Vetter and Z. Jianmin for their generous gift of Fz2 morpholinos, for providing suggestions and sharing their unpublished data. We are grateful to F. van Horck for help with the morpholino-injection technique. We thank B. Harris and I. Iordanova for helpful discussions and J. Borrell for providing help with statistical analysis. We thank I. Dompablo and C. Capitan for excellent technical assistance. This study was supported by grants from the Spanish Ministerio de Educación y Ciencia (BFU-2004-01585), the European Union (QLG3-CT-2001-01460) the Comunidad Autónoma de Madrid (08.5/0049.1/2003) and the Institutional RED CIEN (G03/06) to P.B. and by a Programme Grant from the Wellcome Trust (UK) to C.H. J.R. was supported by a Glaxo–Consejo Superior de Investigaciones Cientificas (CSIC) predoctoral fellowship, a European Molecular Biology Organization (EMBO) short-term fellowship and a 'Development' traveling award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Expression and purification of SFRP1 and its CRD and NTR domains. (PDF 154 kb)
Supplementary Fig. 2
Schematic representation of the Xenopus visual trajectory. (PDF 162 kb)
Supplementary Fig. 3
SFRP1 does not interfere with Wnt-induced neurite outgrowth in chick RGC. (PDF 117 kb)
Supplementary Fig. 4
SFRP1 mediated neurite outgrowth requires the activation of G proteins. (PDF 82 kb)
Rights and permissions
About this article
Cite this article
Rodriguez, J., Esteve, P., Weinl, C. et al. SFRP1 regulates the growth of retinal ganglion cell axons through the Fz2 receptor. Nat Neurosci 8, 1301–1309 (2005). https://doi.org/10.1038/nn1547
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1547
This article is cited by
-
Wnt3a is a promising target in colorectal cancer
Medical Oncology (2023)
-
Morphological neurite changes induced by porcupine inhibition are rescued by Wnt ligands
Cell Communication and Signaling (2021)
-
Secreted frizzled related-protein 2 (Sfrp2) deficiency decreases adult skeletal stem cell function in mice
Bone Research (2021)
-
Wnt signaling in breast cancer: biological mechanisms, challenges and opportunities
Molecular Cancer (2020)
-
A possible founder mutation in FZD6 gene in a Turkish family with autosomal recessive nail dysplasia
BMC Medical Genetics (2019)