Abstract
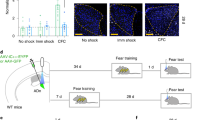
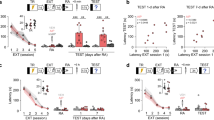
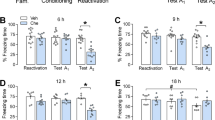
Reconsolidation—the stabilization of a memory after retrieval—is hypothesized to be a critical and distinct component of memory processing, the disruption of which results in memory impairment. In the rat, we found that activation of amygdalar protein kinase A (PKA) was sufficient to enhance memory only when it was retrieved; in contrast, PKA inhibition impaired reconsolidation. This study demonstrates both a selective enhancement and an impairment of memory reconsolidation dependent on amygdalar PKA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
02 May 2006
Replaced Supplementary Methods file
Notes
NOTE: In the version of this article intially published online, the units for some of the values in the Supplementary Methods incorrectly said L. The correct unit should be µM. This error has been corrected.
References
Sara, S.J. Nat. Rev. Neurosci. 1, 212–213 (2000).
Nader, K., Schafe, G.E. & LeDoux, J.E. Nature 406, 722–726 (2000).
Duvarci, S. & Nader, K. J. Neurosci. 24, 9269–9275 (2004).
Gordon, W.C. Physiol. Behav. 19, 95–99 (1977).
Schafe, G.E. & LeDoux, J.E. J. Neurosci. 20, RC96 (2000).
Jentsch, J.D., Olausson, P., Nestler, E.J. & Taylor, J.R. Biol. Psychiatry 52, 111–118 (2002).
Christensen, A.E. et al. J. Biol. Chem. 278, 35394–35402 (2003).
Paxinos, G. & Watson, C. The Rat Brain Atlas in Stereotaxic Co-ordinates 4th edn. (Academic, Sydney, 1996).
Pedreira, M.E. & Maldonado, H. Neuron 38, 863–869 (2003).
Suzuki, A. et al. J. Neurosci. 24, 4787–4795 (2004).
Bahar, A., Dorfman, N. & Dudai, Y. Eur. J. Neurosci. 19, 1115–1118 (2004).
Acknowledgements
We thank J.J. Quinn for her comments on the manuscript. This work was supported by grant DA15222 from the US National Institute on Drug Abuse and grant MH25642 from the National Institute of Mental Health. N.C.T. was supported in part by a Robert F. Leylan Yale University Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Schematic representation of injector placements in basolateral amygdala (BLA). (PDF 934 kb)
Supplementary Fig. 2
PKA activation in the basolateral amygdala does not affect freezing to the reactivation context. (PDF 195 kb)
Supplementary Fig. 3
Inhibition of PKA in the basolateral amygdala does not affect freezing to the reactivation context. (PDF 123 kb)
Rights and permissions
About this article
Cite this article
Tronson, N., Wiseman, S., Olausson, P. et al. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat Neurosci 9, 167–169 (2006). https://doi.org/10.1038/nn1628
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1628
This article is cited by
-
Phosphodiesterase 4 inhibition after retrieval switches the memory fate favoring extinction instead of reconsolidation
Scientific Reports (2023)
-
Forgetting as a form of adaptive engram cell plasticity
Nature Reviews Neuroscience (2022)
-
Sleep enhances reconsolidation-based strengthening of visuospatial memories
Scientific Reports (2022)
-
Sleep’s role in updating aversive autobiographical memories
Translational Psychiatry (2022)
-
Activation of acid‐sensing ion channels by carbon dioxide regulates amygdala synaptic protein degradation in memory reconsolidation
Molecular Brain (2021)