Abstract
Motor function requires that motor axons extend from the spinal cord at regular intervals and that they are myelinated by Schwann cells. Little attention has been given to another cellular structure, the perineurium, which ensheaths the motor nerve, forming a flexible, protective barrier. Consequently, the origin of perineurial cells and their roles in motor nerve formation are poorly understood. Using time-lapse imaging in zebrafish, we show that perineurial cells are born in the CNS, arising as ventral spinal-cord glia before migrating into the periphery. In embryos lacking perineurial glia, motor neurons inappropriately migrated outside of the spinal cord and had aberrant axonal projections, indicating that perineurial glia carry out barrier and guidance functions at motor axon exit points. Additionally, reciprocal signaling between perineurial glia and Schwann cells was necessary for motor nerve ensheathment by both cell types. These insights reveal a new class of CNS-born glia that critically contributes to motor nerve development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Vermeren, M. et al. Integrity of developing spinal motor columns is regulated by neural crest derivatives at motor exit points. Neuron 37, 403–415 (2003).
Akert, K., Sandri, C., Weibel, E.R., Peper, K. & Moor, H. The fine structure of the perineural endothelium. Cell Tissue Res. 165, 281–295 (1976).
Allt, G. Ultrastructural features of the immature peripheral nerve. J. Anat. 105, 283–293 (1969).
Bourne, G.H. The Structure and Function of Nervous Tissue Vol. V (Academic Press, New York, 1968).
Burkel, W.E. The histological fine structure of perineurium. Anat. Rec. 158, 177–189 (1967).
Haller, F.R. & Low, F.N. The fine structure of the peripheral nerve root sheath in the subarachnoid space in the rat and other laboratory animals. Am. J. Anat. 131, 1–19 (1971).
Nordlander, R.H., Singer, J.F., Beck, R. & Singer, M. An ultrastructural examination of early ventral root formation in amphibia. J. Comp. Neurol. 199, 535–551 (1981).
Guyton, A.C. Structure and Function of the Nervous System. Ch. 14 254 (Saunders, Philadelphia, 1972).
Kristensson, K. & Olsson, Y. The perineurium as a diffusion barrier to protein tracers. Differences between mature and immature animals. Acta Neuropathol. (Berl.) 17, 127–138 (1971).
Olsson, Y. Microenvironment of the peripheral nervous system under normal and pathological conditions. Crit. Rev. Neurobiol. 5, 265–311 (1990).
Du Plessis, D.G., Mouton, Y.M., Muller, C.J. & Geiger, D.H. An ultrastructural study of the development of the chicken perineurial sheath. J. Anat. 189, 631–641 (1996).
Parmantier, E. et al. Schwann cell–derived Desert hedgehog controls the development of peripheral nerve sheaths. Neuron 23, 713–724 (1999).
Schmidt, H. et al. The embryonic central nervous system lineages of Drosophila melanogaster. II. Neuroblast lineages derived from the dorsal part of the neuroectoderm. Dev. Biol. 189, 186–204 (1997).
Klambt, C. & Goodman, C.S. The diversity and pattern of glia during axon pathway formation in the Drosophila embryo. Glia 4, 205–213 (1991).
Parker, R.J. & Auld, V.J. Roles of glia in the Drosophila nervous system. Semin. Cell Dev. Biol. 17, 66–77 (2006).
Sepp, K.J., Schulte, J. & Auld, V.J. Developmental dynamics of peripheral glia in Drosophila melanogaster. Glia 30, 122–133 (2000).
Sepp, K.J., Schulte, J. & Auld, V.J. Peripheral glia direct axon guidance across the CNS/PNS transition zone. Dev. Biol. 238, 47–63 (2001).
Schafer, M., Kinzel, D. & Winkler, C. Discontinuous organization and specification of the lateral floor plate in zebrafish. Dev. Biol. 301, 117–129 (2007).
Myers, P.Z., Eisen, J.S. & Westerfield, M. Development and axonal outgrowth of identified motoneurons in the zebrafish. J. Neurosci. 6, 2278–2289 (1986).
Kirby, B.B. et al. In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat. Neurosci. 9, 1506–1511 (2006).
Ng, A.N. et al. Formation of the digestive system in zebrafish. III. Intestinal epithelium morphogenesis. Dev. Biol. 286, 114–135 (2005).
Shin, J., Park, H.C., Topczewska, J.M., Mawdsley, D.J. & Appel, B. Neural cell fate analysis in zebrafish using olig2 BAC transgenics. Methods Cell Sci. 25, 7–14 (2003).
Carney, T.J. et al. A direct role for Sox10 in specification of neural crest–derived sensory neurons. Development 133, 4619–4630 (2006).
Lyons, D.A. et al. erbb3 and erbb2 are essential for schwann cell migration and myelination in zebrafish. Curr. Biol. 15, 513–524 (2005).
Ono, F., Mandel, G. & Brehm, P. Acetylcholine receptors direct rapsyn clusters to the neuromuscular synapse in zebrafish. J. Neurosci. 24, 5475–5481 (2004).
Odenthal, J. & Nusslein-Volhard, C. Fork head domain genes in zebrafish. Dev. Genes Evol. 208, 245–258 (1998).
Schauerte, H.E. et al. Sonic hedgehog is not required for the induction of medial floor plate cells in the zebrafish. Development 125, 2983–2993 (1998).
Incardona, J.P., Gaffield, W., Kapur, R.P. & Roelink, H. The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development 125, 3553–3562 (1998).
Cooper, M.K., Porter, J.A., Young, K.E. & Beachy, P.A. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science 280, 1603–1607 (1998).
Dutton, K.A. et al. Zebrafish colourless encodes sox10 and specifies non-ectomesenchymal neural crest fates. Development 128, 4113–4125 (2001).
Britsch, S. et al. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 15, 66–78 (2001).
Kuhlbrodt, K., Herbarth, B., Sock, E., Hermans-Borgmeyer, I. & Wegner, M. Sox10, a novel transcriptional modulator in glial cells. J. Neurosci. 18, 237–250 (1998).
Paratore, C., Goerich, D.E., Suter, U., Wegner, M. & Sommer, L. Survival and glial fate acquisition of neural crest cells are regulated by an interplay between the transcription factor Sox10 and extrinsic combinatorial signaling. Development 128, 3949–3961 (2001).
Gamble, H.J. & Eames, R.A. An electron microscope study of the connective tissues of human peripheral nerve. J. Anat. 98, 655–663 (1964).
Bunge, M.B., Wood, P.M., Tynan, L.B., Bates, M.L. & Sanes, J.R. Perineurium originates from fibroblasts: demonstration in vitro with a retroviral marker. Science 243, 229–231 (1989).
Joseph, N.M. et al. Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development 131, 5599–5612 (2004).
Raven, P. Experiments on the origin of the sheath cells and sympathetic neuroblasts in amphibia. J. Comp. Neurol. 67, 221–240 (1937).
Jones, D.S. The origin of the sympathetic trunk in the chick embryo. Anat. Rec. 70, 45–65 (1937).
Weston, J.A. A radioautographic analysis of the migration and localization of trunk neural crest cells in the chick. Dev. Biol. 6, 279–310 (1963).
Dickinson, D.P., Machnicki, M., Ali, M.M., Zhang, Z. & Sohal, G.S. Ventrally emigrating neural tube (VENT) cells: a second neural tube–derived cell population. J. Anat. 205, 79–98 (2004).
Yaneza, M., Gilthorpe, J.D., Lumsden, A. & Tucker, A.S. No evidence for ventrally migrating neural tube cells from the mid- and hindbrain. Dev. Dyn. 223, 163–167 (2002).
Boot, M.J., Gittenberger-de Groot, A.C., van Iperen, L. & Poelmann, R.E. The myth of ventrally emigrating neural tube (VENT) cells and their contribution to the developing cardiovascular system. Anat. Embryol. (Berl.) 206, 327–333 (2003).
Freeman, M.R. & Doherty, J. Glial cell biology in Drosophila and vertebrates. Trends Neurosci. 29, 82–90 (2006).
Ungos, J.M., Karlstrom, R.O. & Raible, D.W. Hedgehog signaling is directly required for the development of zebrafish dorsal root ganglia neurons. Development 130, 5351–5362 (2003).
Berger, P., Niemann, A. & Suter, U. Schwann cells and the pathogenesis of inherited motor and sensory neuropathies (Charcot-Marie-Tooth disease). Glia 54, 243–257 (2006).
Meyer Zu Hörste, G. & Nave, K.A. Animal models of inherited neuropathies. Curr. Opin. Neurol. 19, 464–473 (2006).
Zuchner, S. & Vance, J.M. Mechanisms of disease: a molecular genetic update on hereditary axonal neuropathies. Nat. Clin. Pract. Neurol. 2, 45–53 (2006).
Macarenco, R.S., Ellinger, F. & Oliveira, A.M. Perineurioma: a distinctive and underrecognized peripheral nerve sheath neoplasm. Arch. Pathol. Lab. Med. 131, 625–636 (2007).
Erlandson, R.A. The enigmatic perineurial cell and its participation in tumors and in tumorlike entities. Ultrastruct. Pathol. 15, 335–351 (1991).
Kelsh, R.N. et al. Zebrafish pigmentation mutations and the processes of neural crest development. Development 123, 369–389 (1996).
Acknowledgements
We thank T. Piotrowski, J. Shin, W. Talbot and R. Karlstrom for reagents and fish, M. Bhat, V. Auld and members of the Appel lab for valuable discussions, and J. Weston for comments on the manuscript. Reagents also were provided by the Zebrafish International Resource Center, supported by grant P40 RR012546 from the US National Institutes of Health (NIH) National Center for Research Resources. This work was supported by the Post Doctoral Training Program in Neurogenomics-MH65215-03 (S.K.), NIH grant R01 NS046668 (B.A.), NIH grant R01 GM054544 (K.B.) and a zebrafish initiative funded by the Vanderbilt University Academic Venture Capital Fund.
Author information
Authors and Affiliations
Contributions
S.K. produced all of the data except for the electron microscopy images shown in Figure 5c,d, which were produced by E.W. N.T. created the Tg(sox10(7.2):mrfp) transgenic line and H.-C.P. created the Tg(olig2:dsred2) transgenic line. B.A. supervised the experiments and wrote the manuscript with S.K. and K.B.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1 and 2, and Figures 1–4 (PDF 950 kb)
Supplementary Video 1
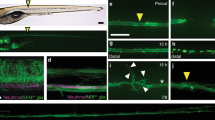
Peripheral nkx2.2a+ cells originate in the CNS. An excerpt from a 24-h time-lapse movie of a Tg(nkx2.2a:megfp) embryo. The images are from a lateral view, focused on the mid-trunk spinal cord. Dorsal is up and anterior is to the left. Sequence shown begins at 40 hpf and ends at approximately 60 hpf. Red circle marks the position where an nkx2.2a+ cell will exit the spinal cord. The migrating cell is outlined in red. Images were collected every 5 min and the movie runs at 3 frames per s. Annotations, including pauses, were added in Openlab (Improvision) before exporting into Quicktime. (MOV 46922 kb)
Supplementary Video 2
Peripheral nkx2.2a+ cells divide and extend into the periphery. Excerpt from a 24-h time-lapse movie of a Tg(nkx2.2a:megfp) embryo. The images are from a lateral view, focused on the mid-trunk spinal cord. Dorsal is up and anterior is to the left. Sequence shown begins at 52 hpf and ends at approximately 64 hpf. The red ellipses mark peripheral nkx2.2a+ cells. Images were collected every 5 min and the movie runs at 3 frames per s. (MOV 50688 kb)
Supplementary Video 3
olig2+ motor axons are ensheathed by nkx2.2a+ cells. Excerpt from a 24-h time-lapse sequence of a Tg(nkx2.2a:megfp);Tg(olig2:dsred2) embryo. The images are from a lateral view, focused on the trunk spinal cord. Dorsal is up and anterior is to the left. Sequence shown begins at 52 hpf and ends at approximately 62 hpf. A red circle marks a motor nerve root. Asterisks mark cells dividing into two. Yellow bars throughout the sequence mark the extension of nkx2.2a+ cells along olig2+ axons (red). Images were collected every 10 min and the movie runs at 3 frames per s. (MOV 23488 kb)
Supplementary Video 4
nkx2.2a+ perineurial glia fail to form sheaths in clsm241 embryos. Excerpt from a 24-h time-lapse sequence of a Tg(nkx2.2a:megfp);clsm241 embryo. The images are from a lateral view, focused on the trunk spinal cord. Dorsal is up and anterior is to the left. Sequence shown begins at 48 hpf and ends at approximately 72 hpf. The red ellipse in the first frame of the sequence marks the position of a developing motor nerve. The first asterisk of the sequence marks the process of a CNS cell (red outline). A second asterisk marks another nkx2.2a+ cell emerging from the spinal cord. Images were collected every 10 min and the movie runs at 3 frames per s. (MOV 48552 kb)
Supplementary Video 5
nkx2.2a+ perineurial glia fail to form sheaths in clstw11 embryos. Excerpt from a 24-h time-lapse sequence of aTg(nkx2.2a:megfp);clstw11 embryo. The images are from a lateral view, focused on the trunk spinal cord. Dorsal is up and anterior is to the left. Sequence shown begins at 48 hpf and ends at approximately 62 hpf. Red circles mark positions of motor nerves. Asterisks label cells emerging from the CNS. Images were collected every 5 min and the movie runs at 3 frames per s. (MOV 49607 kb)
Rights and permissions
About this article
Cite this article
Kucenas, S., Takada, N., Park, HC. et al. CNS-derived glia ensheath peripheral nerves and mediate motor root development. Nat Neurosci 11, 143–151 (2008). https://doi.org/10.1038/nn2025
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn2025
This article is cited by
-
Developmental exposure to domoic acid targets reticulospinal neurons and leads to aberrant myelination in the spinal cord
Scientific Reports (2023)
-
Developmental independence of median fins from the larval fin fold revises their evolutionary origin
Scientific Reports (2022)
-
Functional in vivo characterization of sox10 enhancers in neural crest and melanoma development
Communications Biology (2021)
-
Sulf2a controls Shh-dependent neural fate specification in the developing spinal cord
Scientific Reports (2021)
-
Metabolic Transporters in the Peripheral Nerve—What, Where, and Why?
Neurotherapeutics (2021)