Abstract
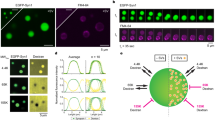
Presynaptic modulation of synaptic transmission provides an important basis for control of synaptic function. The synapsins, a family of highly conserved proteins associated with synaptic vesicles, have long been implicated in the regulation of neurotransmitter release. However, direct physiological measurements of the molecular mechanisms have been lacking. Here we show that in living hippocampal terminals, green fluorescent protein (GFP)-labeled synapsin Ia dissociates from synaptic vesicles, disperses into axons during action potential (AP) firing, and reclusters to synapses after the cessation of synaptic activity. Using various mutated forms of synapsin Ia that prevent phosphorylation at specific sites, we performed simultaneous FM 4-64 measurements of vesicle pool mobilization along with synapsin dispersion kinetics. These studies indicate that the rate of synapsin dispersion is controlled by phosphorylation, which in turn controls the kinetics of vesicle pool turnover. Thus synapsin acts as a phosphorylation-state-dependent regulator of synaptic vesicle mobilization, and hence, neurotransmitter release.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Greengard, P., Valtorta, F., Czernik, A. J. & Benfenati, F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science 259, 780–785 (1993).
Hilfiker, S. et al. Two sites of action for synapsin domain E in regulating neurotransmitter release. Nat. Neurosci. 1, 29–35 (1998).
Hosaka, M. & Sudhof, T. C. Synapsin III, a novel synapsin with an unusual regulation by Ca2+. J. Biol. Chem. 273, 13371–13374 (1998).
Kao, H. T. et al. A third member of the synapsin gene family. Proc. Natl. Acad. Sci. USA 95, 4667–4672 (1998).
Kao, H. T. et al. Molecular evolution of the synapsin gene family. J. Exp. Zool. 285, 360–377 (1999).
Sudhof, T. C. et al. Synapsins: mosaics of shared and individual domains in a family of synaptic vesicle phosphoproteins. Science 245, 1474–1480 (1989).
Huttner, W. B., Schiebler, W., Greengard, P. & De Camilli, P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J. Cell Biol. 96, 1374–1388 (1983).
De Camilli, P., Harris, S. M. Jr., Huttner, W. B. & Greengard, P. Synapsin I (Protein I), a nerve terminal-specific phosphoprotein. II. Its specific association with synaptic vesicles demonstrated by immunocytochemistry in agarose-embedded synaptosomes. J. Cell Biol. 96, 1355–1373 (1983).
Mandell, J. W. et al. Synapsins in the vertebrate retina: absence from ribbon synapses and heterogeneous distribution among conventional synapses. Neuron 5, 19–33 (1990).
Mandell, J. W., Czernik, A. J., De Camilli, P., Greengard, P. & Townes-Anderson, E. Differential expression of synapsins I and II among rat retinal synapses. J. Neurosci. 12, 1736–1749 (1992).
Bahler, M. & Greengard, P. Synapsin I bundles F-actin in a phosphorylation-dependent manner. Nature 326, 704–707 (1987).
Bahler, M., Benfenati, F., Valtorta, F., Czernik, A. J. & Greengard, P. Characterization of synapsin I fragments produced by cysteine-specific cleavage: a study of their interactions with F-actin. J. Cell Biol. 108, 1841–1849 (1989).
Benfenati, F., Valtorta, F., Bahler, M. & Greengard, P. Synapsin I, a neuron-specific phosphoprotein interacting with small synaptic vesicles and F-actin. Cell Biol. Int. Rep. 13, 1007–1021 (1989).
Benfenati, F., Bahler, M., Jahn, R. & Greengard, P. Interactions of synapsin I with small synaptic vesicles: distinct sites in synapsin I bind to vesicle phospholipids and vesicle proteins. J. Cell Biol. 108, 1863–1872 (1989).
Benfenati, F., Greengard, P., Brunner, J. & Bahler, M. Electrostatic and hydrophobic interactions of synapsin I and synapsin I fragments with phospholipid bilayers. J. Cell Biol. 108, 1851–1862 (1989).
Benfenati, F., Valtorta, F., Chieregatti, E. & Greengard, P. Interaction of free and synaptic vesicle-bound synapsin I with F-actin. Neuron 8, 377–386 (1992).
Benfenati, F. et al. Interactions of synapsin I with phospholipids: possible role in synaptic vesicle clustering and in the maintenance of bilayer structures. J. Cell Biol. 123, 1845–1855 (1993).
Ceccaldi, P. E. et al. Dephosphorylated synapsin I anchors synaptic vesicles to actin cytoskeleton: an analysis by videomicroscopy. J. Cell Biol. 128, 905–912 (1995).
Chilcote, T. J., Siow, Y. L., Schaeffer, E., Greengard, P. & Thiel, G. Synapsin IIa bundles actin filaments. J. Neurochem. 63, 1568–1571 (1994).
Greengard, P., Browning, M. D., McGuinness, T. L. & Llinas, R. Synapsin I, a phosphoprotein associated with synaptic vesicles: possible role in regulation of neurotransmitter release. Adv. Exp. Med. Biol. 221, 135–153 (1987).
Hosaka, M., Hammer, R. E. & Sudhof, T. C. A phospho-switch controls the dynamic association of synapsins with synaptic vesicles. Neuron 24, 377–387 (1999).
Thiel, G., Sudhof, T. C. & Greengard, P. Synapsin II. Mapping of a domain in the NH2-terminal region which binds to small synaptic vesicles. J. Biol. Chem. 265, 16527–16533 (1990).
Schiebler, W., Jahn, R., Doucet, J. P., Rothlein, J. & Greengard, P. Characterization of synapsin I binding to small synaptic vesicles. J. Biol. Chem. 261, 8383–8390 (1986).
De Camilli, P., Benfenati, F., Valtorta, F. & Greengard, P. The synapsins. Annu. Rev. Cell Biol. 6, 433–460 (1990).
Li, L. et al. Impairment of synaptic vesicle clustering and of synaptic transmission, and increased seizure propensity, in synapsin I-deficient mice. Proc. Natl. Acad. Sci. USA 92, 9235–9239 (1995).
Rosahl, T. W. et al. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature 375, 488–493 (1995).
Rosahl, T. W. et al. Short-term synaptic plasticity is altered in mice lacking synapsin I. Cell 75, 661–670 (1993).
Stefani, G. et al. Kinetic analysis of the phosphorylation-dependent interactions of synapsin I with rat brain synaptic vesicles. J. Physiol. (Lond.) 504, 501–515 (1997).
Sihra, T. S., Wang, J. K., Gorelick, F. S. & Greengard, P. Translocation of synapsin I in response to depolarization of isolated nerve terminals. Proc. Natl. Acad. Sci. USA 86, 8108–8112 (1989).
Torri, T. F., Bossi, M., Fesce, R., Greengard, P. & Valtorta, F. Synapsin I partially dissociates from synaptic vesicles during exocytosis induced by electrical stimulation. Neuron 9, 1143–1153 (1992).
Henkel, A. W. & Betz, W. J. Staurosporine blocks evoked release of FM1-43 but not acetylcholine from frog motor nerve terminals. J. Neurosci. 15, 8246–8258 (1995).
Sankaranarayanan, S. & Ryan, T. A. Real-time measurements of vesicle-SNARE recycling in synapses of the central nervous system. Nat. Cell Biol. 2, 197–204 (2000).
Czernik, A. J., Pang, D. T. & Greengard, P. Amino acid sequences surrounding the cAMP-dependent and calcium/calmodulin-dependent phosphorylation sites in rat and bovine synapsin I. Proc. Natl. Acad. Sci. USA 84, 7518–7522 (1987).
Llinas, R., McGuinness, T. L., Leonard, C. S., Sugimori, M. & Greengard, P. Intraterminal injection of synapsin I or calcium/calmodulin-dependent protein kinase II alters neurotransmitter release at the squid giant synapse. Proc. Natl. Acad. Sci. USA 82, 3035–3039 (1985).
Llinas, R., Gruner, J. A., Sugimori, M., McGuinness, T. L. & Greengard, P. Regulation by synapsin I and Ca2+-calmodulin-dependent protein kinase II of the transmitter release in squid giant synapse. J. Physiol. (Lond.) 436, 257–282 (1991).
Hosaka, M. & Sudhof, T. C. Homo- and heterodimerization of synapsins. J. Biol. Chem. 274, 16747–16753 (1999).
Tanaka, H. et al. Molecular modification of N-cadherin in response to synaptic activity. Neuron 25, 93–107 (2000).
Ryan, T. A., Li, L., Chin, L. S., Greengard, P. & Smith, S. J. Synaptic vesicle recycling in synapsin I knock-out mice. J. Cell Biol. 134, 1219–1227 (1996).
Esser, L. et al. Synapsin I is structurally similar to ATP-utilizing enzymes. EMBO J. 17, 977–984 (1998).
Hosaka, M. & Sudhof, T. C. Synapsins I and II are ATP-binding proteins with differential Ca2+ regulation. J. Biol. Chem. 273, 1425–1429 (1998).
Ferreira, A. et al. Distinct roles of synapsin I and synapsin II during neuronal development. Mol. Med. 4, 22–28 (1998).
Ryan, T. A. Inhibitors of myosin light chain kinase block synaptic vesicle pool mobilization during action potential firing. J. Neurosci. 19, 1317–1323 (1999).
Threadgill, R., Bobb, K. & Ghosh, A. Regulation of dendritic growth and remodeling by Rho, Rac, and Cdc42. Neuron 19, 625–634 (1997).
Acknowledgements
We would like to thank R. Scheller for providing the GFP-VAMP construct, W. Yan for technical assistance and members of the Ryan and Greengard labs for discussions. This work was supported by grants from the NIH to T.A.R. (NS24692 and GM61925-01) and P.G. (MH39327). T.A.R. is an Alfred P. Sloan Research Fellow.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chi, P., Greengard, P. & Ryan, T. Synapsin dispersion and reclustering during synaptic activity. Nat Neurosci 4, 1187–1193 (2001). https://doi.org/10.1038/nn756
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn756
This article is cited by
-
Interactions of calmodulin kinase II with the dopamine transporter facilitate cocaine-induced enhancement of evoked dopamine release
Translational Psychiatry (2023)
-
Crosstalk of Synapsin1 palmitoylation and phosphorylation controls the dynamicity of synaptic vesicles in neurons
Cell Death & Disease (2022)
-
Ca2+ binding to synapsin I regulates resting Ca2+ and recovery from synaptic depression in nerve terminals
Cellular and Molecular Life Sciences (2022)
-
Phase separation at the synapse
Nature Neuroscience (2020)
-
Early Synaptic Alterations and Selective Adhesion Signaling in Hippocampal Dendritic Zones Following Organophosphate Exposure
Scientific Reports (2019)