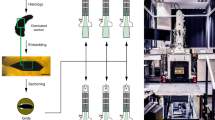
Abstract
Serial section electron microscopy is typically applied to investigation of small tissue volumes encompassing subcellular structures. However, in neurobiology, the need to relate subcellular structure to organization of neural circuits can require investigation of large tissue volumes at ultrastructural resolution. Analysis of ultrastructure and three-dimensional reconstruction of even one to a few cells is time consuming, and still does not generate the necessary numbers of observations to form well-grounded insights into biological principles. We describe an assemblage of existing computer-based methods and strategies for graphical analysis of large photographic montages to accomplish the study of multiple neurons through large tissue volumes. Sample preparation, data collection and subsequent analyses can be completed within 3–4 months. These methods generate extremely large data sets that can be mined in future studies of nervous system organization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others

References
Campbell, P.K., Jones, K.E., Huber, R.J., Horch, K.W. & Normann, R.A. A silicon-based, three-dimensional neural interface: manufacturing processes for an intracortical electrode array. IEEE Trans. Biomed. Eng. 38, 758–768 (1991).
Hoogerwerf, A.C. & Wise, K.D. A three-dimensional microelectrode array for chronic neural recording. IEEE Trans. Biomed. Eng. 41, 1136–1146 (1994).
Spirou, G.A., Rager, J. & Manis, P.B. Convergence of auditory-nerve fiber projections onto globular bushy cells. Neuroscience 136, 843–863 (2005).
Fiala, J.C., Feinberg, M., Popov, V. & Harris, K.M. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J. Neurosci. 18, 8900–8911 (1998).
Fiala, J.C. & Harris, K.M. Extending unbiased stereology of brain ultrastructure to three-dimensional volumes. J. Am. Med. Inform. Assoc. 8, 1–16 (2001).
Woolf, T.B., Shepherd, G.M. & Greer, C.A. Serial reconstructions of granule cell spines in the mammalian olfactory bulb. Synapse 7, 181–192 (1991).
Wilson, C.J., Groves, P.M., Kitai, S.T. & Linder, J.C. Three-dimensional structure of dendritic spines in the rat neostriatum. J. Neurosci. 3, 383–388 (1983).
Harris, K.M. & Stevens, J.K. Dendritic spines of rat cerebellar Purkinje cells: serial electron microscopy with reference to their biophysical characteristics. J. Neurosci. 8, 4455–4469 (1988).
Famiglietti, E.V. Synaptic organization of starburst amacrine cells in rabbit retina: analysis of serial thin sections by electron microscopy and graphic reconstruction. J. Comp. Neurol. 309, 40–70 (1991).
Nicol, M.J. & Walmsley, B. Ultrastructural basis of synaptic transmission between endbulbs of Held and bushy cells in the rat cochlear nucleus. J. Physiol. 539, 713–723 (2002).
Satzler, K. et al. Three-dimensional reconstruction of a calyx of Held and its postsynaptic principal neuron in the medial nucleus of the trapezoid body. J. Neurosci. 22, 10567–10579 (2002).
Kosaka, T. Synapses in the granule cell layer of the rat dentate gyrus: serial-sectioning study. Exp. Brain Res. 112, 237–243 (1996).
Gibbins, I.L., Rodgers, H.F., Matthew, S.E. & Murphy, S.M. Synaptic organisation of lumbar sympathetic ganglia of guinea pigs: serial section ultrastructural analysis of dye-filled sympathetic final motor neurons. J. Comp. Neurol. 402, 285–302 (1998).
Ramón y Cajal, S. Histologie du Système Nerveux de l'Homme & des Vertébrés. Madrid (Spain): Instituto Ramón y Cajal 1955 (1909).
White, J.G., Southgate, E., J.N., T. & Brenner, S. The structure of the nervous-system of the nematode Caenorhabditis-elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 314, 1–340 (1986).
Lorente de No, R. The Primary Acoustic Nuclei (Raven Press, New York, 1981).
Ryugo, D.K. & Sento, S. Synaptic connections of the auditory nerve in cats: relationship between endbulbs of held and spherical bushy cells. J. Comp. Neurol. 305, 35–48 (1991).
Cant, N.B. & Morest, D.K. The bushy cells in the anteroventral cochlear nucleus of the cat. A study with the electron microscope. Neuroscience 4, 1925–1945 (1979).
Tolbert, L.P., Morest, D.K. & Yurgelun-Todd, D.A. The neuronal architecture of the anteroventral cochlear nucleus of the cat in the region of the cochlear nerve root: horseradish peroxidase labelling of identified cell types. Neuroscience 7, 3031–3052 (1982).
Held, H. Die centrale Gehorleitung. Archiv. Anat. Physiol. Anat. Abt 17, 201–248 (1893).
Morest, D.K. The growth of synaptic endings in the mammalian brain: a study of the calyces of the trapezoid body. Z. Anat. Entwicklungsgesch. 127, 201–220 (1968).
Berrebi, A.S. & Spirou, G.A. PEP-19 immunoreactivity in the cochlear nucleus and superior olive of the cat. Neuroscience 83, 535–554 (1998).
Hoffpauir, B.K., Grimes, J.L., Mathers, P.H. & Spirou, G.A. Synaptogenesis of the calyx of Held: rapid onset of function and one-to-one morphological innervation. J. Neurosci. 26, 5511–5523 (2006).
Kremer, J.R., Mastronarde, D.N. & McIntosh, J.R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 116, 71–76 (1996).
Hessler, D. et al. Programs for visualization in three-dimensional microscopy. Neuroimage 1, 55–67 (1992).
Fiala, J.C. Reconstruct a free editor for serial section microscopy. J. Microsc. 218, 52–61 (2005).
Chow, S.K. et al. Automated microscopy system for mosaic acquisition and processing. J. Microsc. 222, 76–84 (2006).
Denk, W. & Horstmann, H. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol. 2, e329 (2004).
Briggman, K.L. & Denk, W. Towards neural circuit reconstruction with volume electron microscopy techniques. Curr. Opin. Neurobiol. 16, 562–570 (2006).
Leighton, S.B. SEM images of block faces, cut by a miniature microtome within the SEM—a technical note. Scan. Electron Microsc. 2, 73–76 (1981).
Sosinsky, G.E. et al. Development of a model for microphysiological simulations: small nodes of ranvier from peripheral nerves of mice reconstructed by electron tomography. Neuroinformatics 3, 133–162 (2005).
Harris, K.M. et al. Uniform serial sectioning for transmission electron microscopy. J. Neurosci. 26, 12101–12103 (2006).
Bozzola, J.J. & Russell, L.D. Electron Microscopy: Principles and Techniques for Biologists (Jones and Bartlett, Sudbury, MA., 1999).
Reid, N. & Beesley, J.E. Sectioning and cryosectioning for electron microscopy. in Practical Methods in Electron Microscopy (ed. Glauert, A.M.) (Elsevier, New York, 1991).
Hayat, M.A. Principles and Techniques of Electron Microscopy: Biological Applications (Cambridge University Press, Cambridge, UK, 2000).
Friedrich, V.L. & Mugnaini, E. Preparation of neural tissues for electron microscopy. in Neuroanatomical Tract Tracing Methods (eds. Heimer, L. & RoBards, M.J.) 345–374 (Plennum Press, New York, 1982).
Mannella, C.A., Marko, M. & Buttle, K. Reconsidering mitochondrial structure: new views of an old organelle. Trends Biochem. Sci. 22, 37–38 (1997).
Soto, G.E. et al. Serial section electron tomography: a method for three-dimensional reconstruction of large structures. Neuroimage 1, 230–243 (1994).
Perkins, G. et al. Electron tomography of neuronal mitochondria: three-dimensional structure and organization of cristae and membrane contacts. J. Struct. Biol. 119, 260–272 (1997).
Wilson, C.J., Mastronarde, D.N., McEwen, B. & Frank, J. Measurement of neuronal surface area using high-voltage electron microscope tomography. Neuroimage 1, 11–22 (1992).
Winslow, J.L., Hollenberg, M.J. & Lea, P.J. Resolution limit of serial sections for 3D reconstruction of tubular cristae in rat liver mitochondria. J. Electron Microsc. Tech. 18, 241–248 (1991).
Peachey, L.D. Thin sections. I. A study of section thickness and physical distortion produced during microtomy. J. Biophys. Biochem. Cytol. 4, 233–242 (1958).
De Groot, D.M. Comparison of methods for the estimation of the thickness of ultrathin tissue sections. J. Microsc. 151, 23–42 (1988).
Small, J.V. in Abstracts Fourth European Regional Conference on Electron Microscopy 1, 609–610 (1968).
Mouton, P.R. Principles and Practices of Unbiased Stereology: An Introduction for Bioscientists (Johns Hopkins University Press, Baltimore, MD, 2002).
Larue, D.T. & Winer, J.A. Postembedding immunocytochemistry of large sections of brain tissue: an improved flat embedding technique. J. Neurosci. Methods 68, 125–132 (1996).
Kandler, K. & Friauf, E. Pre- and postnatal development of efferent connections of the cochlear nucleus in the rat. J. Comp. Neurol. 328, 161–184 (1993).
Kil, J., Kageyama, G.H., Semple, M.N. & Kitzes, L.M. Development of ventral cochlear nucleus projections to the superior olivary complex in gerbil. J. Comp. Neurol. 353, 317–340 (1995).
Acknowledgements
This work was supported by NIH/NIDCD (DC005035) and an NIH/NCRR COBRE grant (P20 RR14474) to the Sensory Neuroscience Research Center. We acknowledge Janet Cyr and Guy Perkins for constructive comments, Albert Berrebi for introducing G.S. to electron microscopy and Erika Hartweig for demonstrating serial section techniques.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Graphical reconstruction of calyces of Held. (PDF 99 kb)
Supplementary Box. 1
Tissue Preparation for Electron Microscopy. (PDF 104 kb)
Supplementary Box. 2
Grid Preparation (6 hours). (PDF 86 kb)
Supplementary Movie
3D reconstruction of a calyx of Held. (MOV 16098 kb)
Rights and permissions
About this article
Cite this article
Hoffpauir, B., Pope, B. & Spirou, G. Serial sectioning and electron microscopy of large tissue volumes for 3D analysis and reconstruction: a case study of the calyx of Held. Nat Protoc 2, 9–22 (2007). https://doi.org/10.1038/nprot.2007.9
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.9
This article is cited by
-
Three-dimensional architecture of podocytes revealed by block-face scanning electron microscopy
Scientific Reports (2015)
-
Dissecting a neuron network: FIB-SEM-based 3D-reconstruction of the visual neuropils in the sea spider Achelia langi (Dohrn, 1881) (Pycnogonida)
BMC Biology (2014)
-
High-contrast en bloc staining of neuronal tissue for field emission scanning electron microscopy
Nature Protocols (2012)
-
Improved biocytin labeling and neuronal 3D reconstruction
Nature Protocols (2012)
-
A protocol for preparing GFP-labeled neurons previously imaged in vivo and in slice preparations for light and electron microscopic analysis
Nature Protocols (2009)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.