Key Points
-
The anaphase promoting complex/cyclosome (APC/C) is a 1.5-MDa ubiquitin ligase complex that initiates sister-chromatid separation and exit from mitosis by targeting cyclin B and securin for destruction by the 26S proteasome.
-
APC/C activity is also indirectly required for DNA replication, because APC/C-mediated cyclin degradation leads to the inactivation of cyclin-dependent kinase-1 (Cdk1), which is a prerequisite for the assembly of pre-replication complexes.
-
APC/C is activated by proteins of the Cdc20/Cdh1 family. The interaction between APC/C and its co-activators is tightly controlled by phosphorylation and is restricted to mitosis and G1 phase.
-
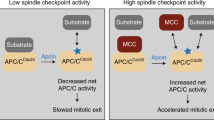
In addition, APC/C activity can be restrained by a number of inhibitory proteins. Mad2 and BubR1 inhibit APC/C during spindle assembly and thereby prevent precocious initiation of anaphase and exit from mitosis. Members of the early mitotic inhibitor-1 (EMI1)/regulator of cyclin A-1 (RCA1) family inhibit APC/C from S phase until early mitosis and during meiosis in vertebrate eggs.
-
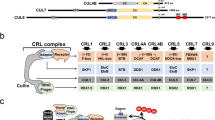
Co-activator proteins activate APC/C by facilitating the recruitment of substrates. All known APC/C co-activators contain a propeller-shaped WD40 domain that interacts with a recognition element in APC/C substrates and is known as the destruction box (D-box).
-
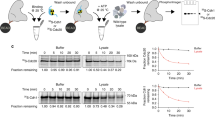
Co-activators are required but not sufficient for substrate recognition because the APC/C subunit Doc1 is also needed for this process. Several observations suggest that the D-box of substrates might interact with both co-activators and APC/C subunits to form a ternary complex in which substrate ubiquitylation occurs.
Abstract
The anaphase promoting complex/cyclosome (APC/C) is a ubiquitin ligase that has essential functions in and outside the eukaryotic cell cycle. It is the most complex molecular machine that is known to catalyse ubiquitylation reactions, and it contains more than a dozen subunits that assemble into a large 1.5-MDa complex. Recent discoveries have revealed an unexpected multitude of mechanisms that control APC/C activity, and have provided a first insight into how this unusual ubiquitin ligase recognizes its substrates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Peters, J. M. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol. Cell 9, 931–943 (2002).
Harper, J. W., Burton, J. L. & Solomon, M. J. The anaphase-promoting complex: it's not just for mitosis any more. Genes Dev. 16, 2179–2206 (2002).
Aristarkhov, A. et al. E2-C, a cyclin-selective ubiquitin carrier protein required for the destruction of mitotic cyclins. Proc. Natl Acad. Sci. USA 93, 4294–4299 (1996).
Yu, H., King, R. W., Peters, J. M. & Kirschner, M. W. Identification of a novel ubiquitin-conjugating enzyme involved in mitotic cyclin degradation. Curr. Biol. 6, 455–466 (1996).
Townsley, F. M., Aristarkhov, A., Beck, S., Hershko, A. & Ruderman, J. V. Dominant-negative cyclin-selective ubiquitin carrier protein E2-C/UbcH10 blocks cells in metaphase. Proc. Natl Acad. Sci. USA 94, 2362–2367 (1997).
Seino, H., Kishi, T., Nishitani, H. & Yamao, F. Two ubiquitin-conjugating enzymes, UbcP1/Ubc4 and UbcP4/Ubc11, have distinct functions for ubiquitination of mitotic cyclin. Mol. Cell. Biol. 23, 3497–3505 (2003).
Mathe, E. et al. The E2-C vihar is required for the correct spatiotemporal proteolysis of cyclin B and itself undergoes cyclical degradation. Curr. Biol. 14, 1723–1733 (2004).
Townsley, F. M. & Ruderman, J. V. Functional analysis of the Saccharomyces cerevisiae UBC11 gene. Yeast 14, 747–757 (1998).
Carroll, C. W. & Morgan, D. O. The Doc1 subunit is a processivity factor for the anaphase-promoting complex. Nature Cell Biol. 4, 880–887 (2002). Shows that the processivity of APC/C-mediated ubiquitylation reactions depends on Doc1.
Deffenbaugh, A. E. et al. Release of ubiquitin-charged Cdc34–S ∼Ub from the RING domain is essential for ubiquitination of the SCFCdc4-bound substrate Sic1. Cell 114, 611–622 (2003).
Schwab, M., Neutzner, M., Mocker, D. & Seufert, W. Yeast Hct1 recognizes the mitotic cyclin Clb2 and other substrates of the ubiquitin ligase APC. EMBO J. 20, 5165–5175 (2001).
Passmore, L. A. et al. Doc1 mediates the activity of the anaphase-promoting complex by contributing to substrate recognition. EMBO J. 22, 786–796 (2003). Shows that co-activators are required but are not sufficient for stable substrate–APC/C interactions, and that Doc1 is also needed for these interactions.
Vodermaier, H. C., Gieffers, C., Maurer-Stroh, S., Eisenhaber, F. & Peters, J. M. TPR subunits of the anaphase-promoting complex mediate binding to the activator protein CDH1. Curr. Biol. 13, 1459–1468 (2003).
Kraft, C., Vodermaier, H. C., Maurer-Stroh, S., Eisenhaber, F. & Peters, J. M. The WD40 propeller domain of Cdh1 functions as a destruction box receptor for APC/C substrates. Mol. Cell 18, 543–553 (2005). Shows that the D-box of APC/C substrates binds directly to the WD40 domain of Cdh1 and provides evidence that this interaction is required for processive substrate ubiquitylation.
Glotzer, M., Murray, A. W. & Kirschner, M. W. Cyclin is degraded by the ubiquitin pathway. Nature 349, 132–138 (1991).
Pfleger, C. M. & Kirschner, M. W. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 14, 655–665 (2000).
Kramer, K. M., Fesquet, D., Johnson, A. L. & Johnston, L. H. Budding yeast RSI1/APC2, a novel gene necessary for initiation of anaphase, encodes an APC subunit. EMBO J. 17, 498–506 (1998).
Yu, H. et al. Identification of a cullin homology region in a subunit of the anaphase-promoting complex. Science 279, 1219–1222 (1998).
Zachariae, W. et al. Mass spectrometric analysis of the anaphase promoting complex from yeast: identification of a subunit related to cullins. Science 279, 1216–1219 (1998).
Tang, Z. et al. APC2 cullin protein and APC11 RING protein comprise the minimal ubiquitin ligase module of the anaphase-promoting complex. Mol. Biol. Cell 12, 3839–3851 (2001).
Gmachl, M., Gieffers, C., Podtelejnikov, A. V., Mann, M. & Peters, J. M. The RING-H2 finger protein APC11 and the E2 enzyme UBC4 are sufficient to ubiquitinate substrates of the anaphase-promoting complex. Proc. Natl Acad. Sci. USA 97, 8973–8978 (2000).
Leverson, J. D. et al. The APC11 RING-H2 finger mediates E2-dependent ubiquitination. Mol. Biol. Cell 11, 2315–2325 (2000).
Passmore, L. A. & Barford, D. Getting into position: the catalytic mechanisms of protein ubiquitylation. Biochem. J. 379, 513–525 (2004).
Petroski, M. D. & Deshaies, R. J. Function and regulation of cullin–RING ubiquitin ligases. Nature Rev. Mol. Cell Biol. 6, 9–20 (2005).
Sudakin, V. et al. The cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity, targets cyclins for destruction at the end of mitosis. Mol. Biol. Cell 6, 185–198 (1995).
Dube, P. et al. Localization of the coactivator Cdh1 and the cullin subunit Apc2 in a cryo-electron microscopy model of vertebrate APC/C. Mol. Cell 20, 867–879 (2005). Provides first insight into where in the vertebrate APC/C structure substrates might be ubiquitylated.
Gieffers, C., Dube, P., Harris, J. R., Stark, H. & Peters, J. M. Three-dimensional structure of the anaphase-promoting complex. Mol. Cell 7, 907–913 (2001).
Passmore, L. A. et al. Structural analysis of the anaphase-promoting complex reveals multiple active sites and insights into polyubiquitylation. Mol. Cell 20, 855–866 (2005). Shows first EM structures of budding yeast APC/C and provides evidence that APC/C might have to dimerize to be fully active.
Schwickart, M. et al. Swm1/Apc13 is an evolutionarily conserved subunit of the anaphase-promoting complex stabilizing the association of Cdc16 and Cdc27. Mol. Cell. Biol. 24, 3562–3576 (2004).
Thornton, B. R. et al. An architectural map of the anaphase-promoting complex. Genes Dev. 20, 449–460 (2006). Provides a detailed map of how APC/C subunits interact with each other.
Schwab, M., Lutum, A. S., Seufert, W. Yeast Hct1 is a regulator of Clb2 cyclin proteolysis. Cell 90, 683–693 (1997).
Visintin, R., Prinz, S. & Amon, A. CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science 278, 460–463 (1997).
Wan, Y. & Kirschner, M. W. Identification of multiple CDH1 homologues in vertebrates conferring different substrate specificities. Proc. Natl Acad. Sci. USA 98, 13066–13071 (2001).
Ohtoshi, A., Maeda, T., Higashi, H., Ashizawa, S. & Hatakeyama, M. Human p55CDC/Cdc20 associates with cyclin A and is phosphorylated by the cyclin A–Cdk2 complex. Biochem. Biophys. Res. Commun. 268, 530–534 (2000).
Burton, J. L. & Solomon, M. J. D box and KEN box motifs in budding yeast Hsl1p are required for APC-mediated degradation and direct binding to Cdc20p and Cdh1p. Genes Dev. 15, 2381–2395 (2001).
Hilioti, Z., Chung, Y. S., Mochizuki, Y., Hardy, C. F. & Cohen-Fix, O. The anaphase inhibitor Pds1 binds to the APC/C-associated protein Cdc20 in a destruction box-dependent manner. Curr. Biol. 11, 1347–1352 (2001).
Pfleger, C. M., Lee, E. & Kirschner, M. W. Substrate recognition by the Cdc20 and Cdh1 components of the anaphase-promoting complex. Genes Dev. 15, 2396–2407 (2001).
Sorensen, C. S. et al. A conserved cyclin-binding domain determines functional interplay between anaphase-promoting complex–Cdh1 and cyclin A–Cdk2 during cell cycle progression. Mol. Cell. Biol. 21, 3692–3703 (2001).
Yamano, H., Gannon, J., Mahbubani, H. & Hunt, T. Cell cycle-regulated recognition of the destruction box of cyclin B by the APC/C in Xenopus egg extracts. Mol. Cell 13, 137–147 (2004). Shows that substrates can interact directly with APC/C in a D-box-dependent manner.
Hayes, M. J. et al. Early mitotic degradation of Nek2A depends on Cdc20-independent interaction with the APC/C. Nature Cell Biol. 8, 607–614 (2006).
Passmore, L. A. & Barford, D. Coactivator functions in a stoichiometric complex with anaphase-promoting complex/cyclosome to mediate substrate recognition. EMBO Rep. 6, 873–878 (2005). Provides the first direct evidence for a ternary complex between APC/C, co-activator and substrate.
Burton, J. L., Tsakraklides, V. & Solomon, M. J. Assembly of an APC–Cdh1–substrate complex is stimulated by engagement of a destruction box. Mol. Cell 18, 533–542 (2005).
Carroll, C. W., Enquist-Newman, M. & Morgan, D. O. The APC subunit Doc1 promotes recognition of the substrate destruction box. Curr. Biol. 15, 11–18 (2005).
Eytan, E., Moshe, Y., Braunstein, I. & Hershko, A. Roles of the anaphase-promoting complex/cyclosome and of its activator Cdc20 in functional substrate binding. Proc. Natl Acad. Sci. USA 103, 2081–2086 (2006).
Wendt, K. S. et al. Crystal structure of the APC10/DOC1 subunit of the human anaphase-promoting complex. Nature Struct. Biol. 8, 784–788 (2001).
Au, S. W., Leng, X., Harper, J. W. & Barford, D. Implications for the ubiquitination reaction of the anaphase-promoting complex from the crystal structure of the Doc1/Apc10 subunit. J. Mol. Biol. 316, 955–968 (2002).
Kominami, K.-i., Seth-Smith, H. & Toda, T. Apc10 and Ste9/Srw1, two regulators of the APC/cyclosome, as well as the CDK inhibitor Rum1 are required for G1-cell cycle arrest in fission yeast. EMBO J. 17, 5388–5399 (1998).
Grossberger, R. et al. Characterization of the DOC1/APC10 subunit of the yeast and the human anaphase-promoting complex. J. Biol. Chem. 274, 14500–14507 (1999).
Gaskell, A., Crennell, S. & Taylor, G. The three domains of a bacterial sialidase: a β-propeller, an immunoglobulin module and a galactose-binding jelly-roll. Structure 3, 1197–1205 (1995).
Irniger, S., Piatti, S., Michaelis, C. & Nasmyth, K. Genes involved in sister chromatid separation are needed for B-type cyclin proteolysis in budding yeast. Cell 81, 269–278 (1995).
King, R. W. et al. A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell 81, 279–288 (1995).
Clute, P. & Pines, J. Temporal and spatial control of cyclin B1 destruction in metaphase. Nature Cell Biol. 1, 82–87 (1999).
Murray, A. W., Solomon, M. J. & Kirschner, M. W. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature 339, 280–286 (1989).
King, R. W., Glotzer, M. & Kirschner, M. W. Mutagenic analysis of the destruction signal of mitotic cyclins and structural characterization of ubiquitinated intermediates. Mol. Biol. Cell 7, 1343–1357 (1996).
Jeffrey, P. D. et al. Mechanism of CDK activation revealed by the structure of a cyclinA–CDK2 complex. Nature 376, 313–320 (1995).
Diffley, J. F. Regulation of early events in chromosome replication. Curr. Biol. 14, R778–R786 (2004).
McGarry, T. J. & Kirschner, M. W. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell 93, 1043–1053 (1998).
Wohlschlegel, J. A. et al. Inhibition of eukaryotic DNA replication by geminin binding to Cdt1. Science 290, 2309–2312 (2000).
Tada, S., Li, A., Maiorano, D., Mechali, M. & Blow, J. J. Repression of origin assembly in metaphase depends on inhibition of RLF-B/Cdt1 by geminin. Nature Cell Biol. 3, 107–113 (2001).
Quinn, L. M., Herr, A., McGarry, T. J. & Richardson, H. The Drosophila Geminin homolog: roles for Geminin in limiting DNA replication, in anaphase and in neurogenesis. Genes Dev. 15, 2741–2754 (2001).
Nasmyth, K. Disseminating the genome: joining, resolving, and separating sister chromatids during mitosis and meiosis. Annu. Rev. Genet. 35, 673–745 (2001).
Yamamoto, A., Guacci, V. & Koshland, D. Pds1p is required for faithful execution of anaphase in the yeast, Saccharomyces cerevisiae. J. Cell Biol. 133, 85–97 (1996).
Jallepalli, P. V. et al. Securin is required for chromosomal stability in human cells. Cell 105, 445–457 (2001).
Mei, J., Huang, X. & Zhang, P. Securin is not required for cellular viability, but is required for normal growth of mouse embryonic fibroblasts. Curr. Biol. 11, 1197–1201 (2001).
Wang, Z., Yu, R. & Melmed, S. Mice lacking pituitary tumor transforming gene show testicular and splenic hypoplasia, thymic hyperplasia, thrombocytopenia, aberrant cell cycle progression, and premature centromere division. Mol. Endocrinol. 15, 1870–1879 (2001).
Stemmann, O., Zou, H., Gerber, S. A., Gygi, S. P. & Kirschner, M. W. Dual inhibition of sister chromatid separation at metaphase. Cell 107, 715–726 (2001).
Gorr, I. H., Boos, D. & Stemmann, O. Mutual inhibition of separase and Cdk1 by two-step complex formation. Mol. Cell 19, 135–141 (2005).
Wirth, K. G. et al. Loss of the anaphase-promoting complex in quiescent cells causes unscheduled hepatocyte proliferation. Genes Dev. 18, 88–98 (2004).
Thornton, B. R. & Toczyski, D. P. Securin and B-cyclin/CDK are the only essential targets of the APC. Nature Cell Biol. 5, 1090–1094 (2003). Describes a strategy to generate yeast strains that can live without otherwise essential APC/C subunits.
Shirayama, M., Toth, A., Galova, M. & Nasmyth, K. APCCdc20 promotes exit from mitosis by destroying the anaphase inhibitor Pds1 and cyclin Clb5. Nature 402, 203–207 (1999).
Shteinberg, M., Protopopov, Y., Listovsky, T., Brandeis, M. & Hershko, A. Phosphorylation of the cyclosome is required for its stimulation by Fizzy/Cdc20. Biochem. Biophys. Res. Commun. 260, 193–198 (1999).
Kramer, E. R., Scheuringer, N., Podtelejnikov, A. V., Mann, M. & Peters, J. M. Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol. Biol. Cell 11, 1555–1569 (2000).
Rudner, A. D. & Murray, A. W. Phosphorylation by Cdc28 activates the Cdc20-dependent activity of the anaphase-promoting complex. J. Cell Biol. 149, 1377–1390 (2000).
Golan, A., Yudkovsky, Y. & Hershko, A. The cyclin-ubiquitin ligase activity of cyclosome/APC is jointly activated by protein kinases Cdk1–cyclin B and Plk. J. Biol. Chem. 277, 15552–15557 (2002).
Kraft, C. et al. Mitotic regulation of the human anaphase-promoting complex by phosphorylation. EMBO J. 22, 6598–6609 (2003).
Zachariae, W., Schwab, M., Nasmyth, K. & Seufert, W. Control of cyclin ubiquitination by CDK-regulated binding of Hct1 to the anaphase promoting complex. Science 282, 1721–1724 (1998).
Jaspersen, S. L., Charles, J. F. & Morgan, D. O. Inhibitory phosphorylation of the APC regulator Hct1 is controlled by the kinase Cdc28 and the phosphatase Cdc14. Curr. Biol. 9, 227–236 (1999).
Blanco, M. A., Sanchez-Diaz, A., de Prada, J. M. & Moreno, S. APCSte9/Srw1 promotes degradation of mitotic cyclins in G1 and is inhibited by Cdc2 phosphorylation. EMBO J. 19, 3945–3955 (2000).
Yamaguchi, S., Okayama, H. & Nurse, P. Fission yeast Fizzy-related protein Srw1p is a G1-specific promoter of mitotic cyclin B degradation. EMBO J. 19, 3968–3977 (2000).
Visintin, R. et al. The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol. Cell 2, 709–718 (1998).
Hagting, A. et al. Human securin proteolysis is controlled by the spindle checkpoint and reveals when the APC/C switches from activation by Cdc20 to Cdh1. J. Cell Biol. 157, 1125–1137 (2002).
Prinz, S., Hwang, E. S., Visintin, R. & Amon, A. The regulation of Cdc20 proteolysis reveals a role for APC components Cdc23 and Cdc27 during S phase and early mitosis. Curr. Biol. 8, 750–760 (1998).
Shirayama, M., Zachariae, W., Ciosk, R. & Nasmyth, K. The Polo-like kinase Cdc5p and the WD-repeat protein Cdc20p/Fizzy are regulators and substrates of the anaphase promoting complex in Saccharomyces cerevisiae. EMBO J. 17, 1336–1349 (1998).
Sorensen, C. S. et al. Nonperiodic activity of the human anaphase-promoting complex-Cdh1 ubiquitin ligase results in continuous DNA synthesis uncoupled from mitosis. Mol. Cell Biol. 20, 7613–7623 (2000).
Huang, J. N., Park, I., Ellingson, E., Littlepage, L. E. & Pellman, D. Activity of the APCCdh1 form of the anaphase-promoting complex persists until S phase and prevents the premature expression of Cdc20p. J. Cell Biol. 154, 85–94 (2001).
Knoblich, J. A. et al. Cyclin E controls S phase progression and its down-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell 77, 107–120 (1994).
Dong, X. et al. Control of G1 in the developing Drosophila eye: Rca1 regulates Cyclin A. Genes Dev. 11, 94–105 (1997).
Grosskortenhaus, R. & Sprenger, F. Rca1 inhibits APC–Cdh1Fzr and is required to prevent cyclin degradation in G2. Dev. Cell 2, 29–40 (2002).
Lukas, C. et al. Accumulation of cyclin B1 requires E2F and cyclin-A-dependent rearrangement of the anaphase-promoting complex. Nature 401, 815–818 (1999).
Hsu, J. Y., Reimann, J. D., Sorensen, C. S., Lukas, J. & Jackson, P. K. E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APCCdh1. Nature Cell Biol. 4, 358–366 (2002).
Reimann, J. D., Gardner, B. E., Margottin-Goguet, F. & Jackson, P. K. Emi1 regulates the anaphase-promoting complex by a different mechanism than Mad2 proteins. Genes Dev. 15, 3278–3285 (2001).
Rape, M. & Kirschner, M. W. Autonomous regulation of the anaphase-promoting complex couples mitosis to S-phase entry. Nature 432, 588–595 (2004).
Yamanaka, A. et al. Cell cycle-dependent expression of mammalian E2-C regulated by the anaphase-promoting complex/cyclosome. Mol. Biol. Cell 11, 2821–2831 (2000).
Benmaamar, R. & Pagano, M. Involvement of the SCF complex in the control of Cdh1 degradation in S-phase. Cell Cycle 4, 1230–1232 (2005).
den Elzen, N. & Pines, J. Cyclin A is destroyed in prometaphase and can delay chromosome alignment and anaphase. J. Cell Biol. 153, 121–136 (2001).
Geley, S. et al. Anaphase-promoting complex/cyclosome-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J. Cell Biol. 153, 137–148 (2001).
Hames, R. S., Wattam, S. L., Yamano, H., Bacchieri, R. & Fry, A. M. APC/C-mediated destruction of the centrosomal kinase Nek2A occurs in early mitosis and depends upon a cyclin A-type D-box. EMBO J. 20, 7117–7127 (2001).
Rieder, C. L., Schultz, A., Cole, R. & Sluder, G. Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J. Cell Biol. 127, 1301–1310 (1994).
Rieder, C. L., Cole, R. W., Khodjakov, A. & Sluder, G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J. Cell Biol. 130, 941–948 (1995).
Musacchio, A. & Hardwick, K. G. The spindle checkpoint: structural insights into dynamic signalling. Nature Rev. Mol. Cell Biol. 3, 731–741 (2002).
Li, Y., Gorbea, C., Mahaffey, D., Rechsteiner, M. & Benezra, R. MAD2 associates with the cyclosome/anaphase-promoting complex and inhibits its activity. Proc. Natl Acad. Sci. USA 94, 12431–12436 (1997).
Fang, G., Yu, H. & Kirschner, M. W. The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation. Genes Dev. 12, 1871–1883 (1998).
Hwang, L. H. et al. Budding yeast Cdc20: a target of the spindle checkpoint. Science 279, 1041–1044 (1998).
Kim, S. H., Lin, D. P., Matsumoto, S., Kitazono, A. & Matsumoto, T. Fission yeast Slp1: an effector of the Mad2-dependent spindle checkpoint. Science 279, 1045–1047 (1998).
Sudakin, V., Chan, G. K. & Yen, T. J. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J. Cell Biol. 154, 925–936 (2001).
Tang, Z., Bharadwaj, R., Li, B. & Yu, H. Mad2-independent inhibition of APCCdc20 by the mitotic checkpoint protein BubR1. Dev. Cell 1, 227–237 (2001).
Fang, G. Checkpoint protein BubR1 acts synergistically with Mad2 to inhibit anaphase-promoting complex. Mol. Biol. Cell 13, 755–766 (2002).
Sironi, L. et al. Mad2 binding to Mad1 and Cdc20, rather than oligomerization, is required for the spindle checkpoint. EMBO J. 20, 6371–6382 (2001).
Luo, X., Tang, Z., Rizo, J. & Yu, H. The Mad2 spindle checkpoint protein undergoes similar major conformational changes upon binding to either Mad1 or Cdc20. Mol. Cell 9, 59–71 (2002).
Sironi, L. et al. Crystal structure of the tetrameric Mad1–Mad2 core complex: implications of a 'safety belt' binding mechanism for the spindle checkpoint. EMBO J. 21, 2496–2506 (2002).
Shah, J. V. et al. Dynamics of centromere and kinetochore proteins; implications for checkpoint signaling and silencing. Curr. Biol. 14, 942–952 (2004).
De Antoni, A. et al. The Mad1–Mad2 complex as a template for Mad2 activation in the spindle assembly checkpoint. Curr. Biol. 15, 214–225 (2005). Proposes an elegant prion-like model for the activation of Mad2 that has the capability to explain previously mysterious observations about the spindle-assembly checkpoint.
Fraschini, R. et al. Bub3 interaction with Mad2, Mad3 and Cdc20 is mediated by WD40 repeats and does not require intact kinetochores. EMBO J. 20, 6648–6659 (2001).
Kallio, M., Weinstein, J., Daum, J. R., Burke, D. J. & Gorbsky, G. J. Mammalian p55CDC mediates association of the spindle checkpoint protein Mad2 with the cyclosome/anaphase-promoting complex, and is involved in regulating anaphase onset and late mitotic events. J. Cell Biol. 141, 1393–1406 (1998).
Wassmann, K. & Benezra, R. Mad2 transiently associates with an APC–p55Cdc complex during mitosis. Proc. Natl Acad. Sci. USA 95, 11193–11198 (1998).
Chan, G. K., Jablonski, S. A., Sudakin, V., Hittle, J. C. & Yen, T. J. Human BUBR1 is a mitotic checkpoint kinase that monitors CENP-E functions at kinetochores and binds the cyclosome/APC. J. Cell Biol. 146, 941–954 (1999).
Morrow, C. J. et al. Bub1 and Aurora B cooperate to maintain BubR1-mediated inhibition of APC/CCdc20. J. Cell Sci. 118, 3639–3652 (2005).
Reimann, J. D. et al. Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell 105, 645–655 (2001).
Hansen, D. V., Loktev, A. V., Ban, K. H. & Jackson, P. K. Plk1 regulates activation of the anaphase promoting complex by phosphorylating and triggering SCFβTrCP-dependent destruction of the APC inhibitor Emi1. Mol. Biol. Cell 15, 5623–5634 (2004).
Moshe, Y., Boulaire, J., Pagano, M. & Hershko, A. Role of Polo-like kinase in the degradation of early mitotic inhibitor 1, a regulator of the anaphase promoting complex/cyclosome. Proc. Natl Acad. Sci. USA 101, 7937–7942 (2004).
Guardavaccaro, D. et al. Control of meiotic and mitotic progression by the F box protein β-Trcp1 in vivo. Dev. Cell 4, 799–812 (2003).
Margottin-Goguet, F. et al. Prophase destruction of Emi1 by the SCFβTrCP/Slimb ubiquitin ligase activates the anaphase promoting complex to allow progression beyond prometaphase. Dev. Cell 4, 813–826 (2003).
Sumara, I. et al. Roles of Polo-like kinase 1 in the assembly of functional mitotic spindles. Curr. Biol. 14, 1712–1722 (2004).
van Vugt, M. A. T. M. et al. Polo-like kinase-1 is required for bipolar spindle formation but is dispensable for anaphase promoting complex/Cdc20 activation and initiation of cytokinesis. J. Biol. Chem. 279, 36841–36854 (2004).
Cooper, K. F., Mallory, M. J., Egeland, D. E. & Strich, R. Ama1p is a meiosis specific regulator of the anaphase promoting complex/cyclosome in yeast. Proc. Natl Acad. Sci. USA 97, 14548–14553 (2000).
Oelschlaegel, T. et al. The yeast APC/C subunit Mnd2 prevents premature sister chromatid separation triggered by the meiosis-specific APC/C–Ama1. Cell 120, 773–788 (2005). Shows that the meiosis-specific co-activator Ama1 is regulated by surprisingly complex mechanisms in budding yeast.
Penkner, A. M., Prinz, S., Ferscha, S. & Klein, F. Mnd2, an essential antagonist of the anaphase-promoting complex during meiotic prophase. Cell 120, 789–801 (2005).
Yoon, H. J. et al. Proteomics analysis identifies new components of the fission and budding yeast anaphase-promoting complexes. Curr. Biol. 12, 2048–2054 (2002).
Hall, M. C., Torres, M. P., Schroeder, G. K. & Borchers, C. H. Mnd2 and Swm1 are core subunits of the Saccharomyces cerevisiae anaphase-promoting complex. J. Biol. Chem. 278, 16698–16705 (2003).
Izawa, D., Goto, M., Yamashita, A., Yamano, H. & Yamamoto, M. Fission yeast Mes1p ensures the onset of meiosis II by blocking degradation of cyclin Cdc13p. Nature 434, 529–533 (2005).
Masui, Y. & Markert, C. Cytoplasmic control of nuclear behaviour during meiotic maturation of frog oocytes. J. Exp. Zool. 177, 129–146 (1971).
Vorlaufer, E. & Peters, J.-M. Regulation of the cyclin B degradation system by an inhibitor of mitotic proteolysis. Mol. Biol. Cell 9, 1817–1831 (1998).
Tunquist, B. J. & Maller, J. L. Under arrest: cytostatic factor (CSF)-mediated metaphase arrest in vertebrate eggs. Genes Dev. 17, 683–710 (2003).
Schwab, M. S. et al. Bub1 is activated by the protein kinase p90Rsk during Xenopus oocyte maturation. Curr. Biol. 11, 141–150 (2001).
Tunquist, B. J., Eyers, P. A., Chen, L. G., Lewellyn, A. L. & Maller, J. L. Spindle checkpoint proteins Mad1 and Mad2 are required for cytostatic factor-mediated metaphase arrest. J. Cell Biol. 163, 1231–1242 (2003).
Reimann, J. D. & Jackson, P. K. Emi1 is required for cytostatic factor arrest in vertebrate eggs. Nature 416, 850–854 (2002).
Ohsumi, K., Koyanagi, A., Yamamoto, T. M., Gotoh, T. & Kishimoto, T. Emi1-mediated M-phase arrest in Xenopus eggs is distinct from cytostatic factor arrest. Proc. Natl Acad. Sci. USA 101, 12531–12536 (2004).
Rauh, N. R., Schmidt, A., Bormann, J., Nigg, E. A. & Mayer, T. U. Calcium triggers exit from meiosis II by targeting the APC/C inhibitor XErp1 for degradation. Nature 437, 1048–1052 (2005). Shows how fertilization of vertebrate eggs inactivates the APC/C inhibitor XErp1 and thereby triggers entry into anaphase II and exit from meiosis.
Schmidt, A. et al. Xenopus polo-like kinase Plx1 regulates XErp1, a novel inhibitor of APC/C activity. Genes Dev. 19, 502–513 (2005).
Tung, J. J. et al. A role for the anaphase-promoting complex inhibitor Emi2/XErp1, a homolog of early mitotic inhibitor 1, in cytostatic factor arrest of Xenopus eggs. Proc. Natl Acad. Sci. USA 102, 4318–4323 (2005).
Shoji, S. et al. Mammalian Emi2 mediates cytostatic arrest and transduces the signal for meiotic exit via Cdc20. EMBO J. 25, 834–845 (2006).
Hansen, D. V., Tung, J. J. & Jackson, P. K. CaMKII and polo-like kinase 1 sequentially phosphorylate the cytostatic factor Emi2/XErp1 to trigger its destruction and meiotic exit. Proc. Natl Acad. Sci. USA 103, 608–613 (2006).
Kashevsky, H. et al. The anaphase promoting complex/cyclosome is required during development for modified cell cycles. Proc. Natl Acad. Sci. USA 99, 11217–11222 (2002).
King, R. W., Deshaies, R. J., Peters, J. M. & Kirschner, M. W. How proteolysis drives the cell cycle. Science 274, 1652–1659 (1996).
Mailand, N. & Diffley, J. F. CDKs promote DNA replication origin licensing in human cells by protecting Cdc6 from APC/C-dependent proteolysis. Cell 122, 915–926 (2005).
Littlepage, L. E. & Ruderman, J. V. Identification of a new APC/C recognition domain, the A box, which is required for the Cdh1-dependent destruction of the kinase Aurora-A during mitotic exit. Genes Dev. 16, 2274–2285 (2002).
Lindon, C. & Pines, J. Ordered proteolysis in anaphase inactivates Plk1 to contribute to proper mitotic exit in human cells. J. Cell Biol. 164, 233–241 (2004).
Rape, M., Reddy, S. K. & Kirschner, M. W. The processivity of multiubiquitination by the APC determines the order of substrate degradation. Cell 124, 89–103 (2006). Provides an elegant mechanistic explanation for the phenomenon that different substrates of APC/CCdh1 are degraded at different times.
Acquaviva, C., Herzog, F., Kraft, C. & Pines, J. The anaphase promoting complex/cyclosome is recruited to centromeres by the spindle assembly checkpoint. Nature Cell Biol. 6, 892–898 (2004).
Tugendreich, S., Tomkiel, J., Earnshaw, W. & Hieter, P. CDC27Hs colocalizes with CDC16Hs to the centrosome and mitotic spindle and is essential for the metaphase to anaphase transition. Cell 81, 261–268 (1995).
Huang, J. & Raff, J. W. The disappearance of cyclin B at the end of mitosis is regulated spatially in Drosophila cells. EMBO J. 18, 2184–2195 (1999).
Raff, J. W., Jeffers, K. & Huang, J. Y. The roles of Fzy/Cdc20 and Fzr/Cdh1 in regulating the destruction of cyclin B in space and time. J. Cell Biol. 157, 1139–1149 (2002).
Wakefield, J. G., Huang, J. Y. & Raff, J. W. Centrosomes have a role in regulating the destruction of cyclin B in early Drosophila embryos. Curr. Biol. 10, 1367–1370 (2000).
Jaquenoud, M., van Drogen, F. & Peter, M. Cell cycle-dependent nuclear export of Cdh1p may contribute to the inactivation of APC/CCdh1. EMBO J. 21, 6515–6526 (2002).
Yudkovsky, Y., Shteinberg, M., Listovsky, T., Brandeis, M. & Hershko, A. Phosphorylation of Cdc20/fizzy negatively regulates the mammalian cyclosome/APC in the mitotic checkpoint. Biochem. Biophys. Res. Commun. 271, 299–304 (2000).
D'Angiolella, V., Mari, C., Nocera, D., Rametti, L. & Grieco, D. The spindle checkpoint requires cyclin-dependent kinase activity. Genes Dev. 17, 2520–2525 (2003).
Pfleger, C. M., Salic, A., Lee, E. & Kirschner, M. W. Inhibition of Cdh1–APC by the MAD2-related protein MAD2L2: a novel mechanism for regulating Cdh1. Genes Dev. 15, 1759–1764 (2001).
Chen, J. & Fang, G. MAD2B is an inhibitor of the anaphase-promoting complex. Genes Dev. 15, 1765–1770 (2001).
Tang, Z., Shu, H., Oncel, D., Chen, S. & Yu, H. Phosphorylation of Cdc20 by Bub1 provides a catalytic mechanism for APC/C inhibition by the spindle checkpoint. Mol. Cell 16, 387–397 (2004).
Chung, E. & Chen, R. H. Phosphorylation of Cdc20 is required for its inhibition by the spindle checkpoint. Nature Cell Biol. 5, 748–753 (2003).
Song, M. S. et al. The tumour suppressor RASSF1A regulates mitosis by inhibiting the APC–Cdc20 complex. Nature Cell Biol. 6, 129–137 (2004).
Casaletto, J. B. et al. Inhibition of the anaphase-promoting complex by the Xnf7 ubiquitin ligase. J. Cell Biol. 169, 61–71 (2005).
Jeganathan, K. B., Malureanu, L. & van Deursen, J. M. The Rae1–Nup98 complex prevents aneuploidy by inhibiting securin degradation. Nature 438, 1036–1039 (2005).
Teodoro, J. G., Heilman, D. W., Parker, A. E. & Green, M. R. The viral protein Apoptin associates with the anaphase-promoting complex to induce G2/M arrest and apoptosis in the absence of p53. Genes Dev. 18, 1952–1957 (2004).
Wiebusch, L., Bach, M., Uecker, R. & Hagemeier, C. Human cytomegalovirus inactivates the G0/G1-APC/C ubiquitin ligase by Cdh1 dissociation. Cell Cycle 4, 1435–1439 (2005).
Kornitzer, D., Sharf, R. & Kleinberger, T. Adenovirus E4orf4 protein induces PP2A-dependent growth arrest in Saccharomyces cerevisiae and interacts with the anaphase-promoting complex/cyclosome. J. Cell Biol. 154, 331–344 (2001).
Zachariae, W., Shin, T. H., Galova, M., Obermaier, B. & Nasmyth, K. Identification of subunits of the anaphase-promoting complex Saccharomyces cerevisiae. Science 274, 1201–1204 (1996).
Acknowledgements
I am grateful to A. Musacchio and members of my group for helpful discussions and to H. Stark and D. Barford for providing images. Research in my laboratory is supported by Boehringer Ingelheim, the European Molecular Biology Organization, the 6th Framework Programme of the European Union via the Integrated Project MitoCheck, and the European Science Foundation and the Austrian Science Fund via the EuroDYNA Programme.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Supplementary information
Supplementary information
Supplementary table 1 (PDF 110 kb)
Related links
Glossary
- Ubiquitin ligase (E3)
-
The third enzyme in a series — the first two are designated ubiquitin-activating (E1) and ubiquitin-conjugating (E2) — that is responsible for the ubiquitylation of target proteins. E3 enzymes provide platforms for binding E2 enzymes and specific substrates, thereby coordinating the ubiquitylation of selected substrates.
- Polyubiquitin chains
-
Protein assemblies that are composed of several copies of the small protein ubiquitin. The ubiquitin residues are covalently attached to each other through isopeptide bonds.
- 26S proteasome
-
A large multisubunit protease complex that selectively degrades multi-ubiquitylated proteins. It contains a 20S particle that carries the catalytic activity and two regulatory 19S particles.
- Cyclin-dependent kinase
-
(Cdk). A protein kinase that has activity that depends on an association with a cyclin subunit. Cdks are essential for DNA replication and entry into mitosis.
- Ubiquitin-activating (E1) enzyme
-
An enzyme that activates the C-terminal glycine residue of the small protein ubiquitin, allowing it to form a high-energy thioester bond to a specific cysteine residue of the E1. E1 then transfers this activated form of ubiquitin onto ubiquitin-conjugating (E2) enzymes.
- Ubiquitin-conjugating (E2) enzyme
-
An enzyme that forms a thioester bond with a ubiquitin residue, which is transferred to the E2 enzyme from ubiquitin-activating (E1) enzyme. E2 uses the high energy from the thioester bond to generate an isopeptide bond between the ubiquitin residue and a lysine residue on a substrate protein.
- SCF
-
A multisubunit ubiquitin ligase complex that is composed of two scaffolding subunits (cullin and Skp1), a RING-finger subunit that binds ubiquitin-conjugating (E2) enzymes and one of many F-box subunits that recruit substrates.
- C-box
-
A sequence element (consensus DRF/YIPXR) that was first found in the N-terminal region of Cdc20. It is conserved in all known APC/C co-activators.
- IR-tail
-
A sequence element (consensus IR) at the extreme C terminus of APC/C co-activators and the APC/C subunit Doc1.
- WD40 domain
-
A propeller-shaped protein domain that is composed of sequence repeats that are ∼40-amino-acid residues long and contain tryptophan (W) and aspartate (D) residues in conserved positions. In most cases, seven WD40 repeats fold into a seven-bladed propeller structure.
- D-box
-
(Destruction-box). A sequence element (consensus RXXLXXXN) that was first discovered in the N terminus of mitotic cyclins that is required for their destruction. D-boxes can be recognized by APC/CCdc20 and by APC/CCdh1.
- KEN-box
-
A sequence element (consensus KEN) that is present in many APC/C substrates. KEN-boxes are preferentially, but not exclusively, recognized by APC/CCdh1.
- Cullin
-
A member of the cullin family of proteins. All cullins are subunits of SCF ubiquitin ligases or APC/C, and they bind to a RING-finger subunit via a conserved cullin domain.
- RING finger
-
A small protein domain that binds two atoms of zinc (consensus CXXCX(9–39)CX(1–3)HX(2–3)C/HXXCX(4–48)CXXC). Many RING-finger domains interact with ubiquitin-conjugating (E2) enzymes and have ubiquitin ligase (E3) activity.
- TPR domain
-
(Tetratrico peptide repeat domain). A 34-amino-acid sequence repeat, clusters of which fold into a helical structure and mediate protein–protein interactions.
- Checkpoint
-
A surveillance mechanism that delays progression through the cell cycle if processes such as DNA replication and spindle assembly have not been completed.
- Kinetochore
-
A large proteinacous structure that assembles on centromeric DNA, binds the plus ends of microtubules and thereby connects chromosomes with spindle poles.
- Quiescence
-
The physiological state of cells that are not in the cell cycle.
Rights and permissions
About this article
Cite this article
Peters, JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol 7, 644–656 (2006). https://doi.org/10.1038/nrm1988
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm1988
This article is cited by
-
Distinct characteristics of the DNA damage response in mammalian oocytes
Experimental & Molecular Medicine (2024)
-
CDK9-55 guides the anaphase-promoting complex/cyclosome (APC/C) in choosing the DNA repair pathway choice
Oncogene (2024)
-
Identification of transcriptome characteristics of granulosa cells and the possible role of UBE2C in the pathogenesis of premature ovarian insufficiency
Journal of Ovarian Research (2023)
-
Genome control by SMC complexes
Nature Reviews Molecular Cell Biology (2023)
-
SIRT1 ubiquitination is regulated by opposing activities of APC/C-Cdh1 and AROS during stress-induced premature senescence
Experimental & Molecular Medicine (2023)