Key Points
-
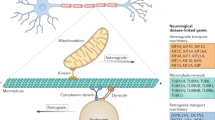
Hereditary neuropathies are genetically heterogeneous and affect neurons and/or Schwann cells. Mutations in several different genes can lead to the same disease phenotype. Conversely, different mutations affecting the same gene can lead to different disease phenotypes.
-
Deletion or duplication of a 1.4 megabase intrachromosomal region on chromosome 17 containing the PMP22 gene causes hereditary neuropathy with liability to pressure palsies or demyelinating Charcot–Marie–Tooth disease (CMT1A), respectively, the most common forms of dominantly inherited demyelinating neuropathy. The deleterious effects of PMP22 gene dosage correlate with the relative amounts of PMP22 protein in compact myelin.
-
Most dominant PMP22 missense mutations that cause disease encode mutant proteins that are retained in the endoplasmic reticulum and/or intermediate compartment. These mutants act by gain of function, and some undergo abnormally prolonged interactions with calnexin, a glycoprotein-specific chaperone.
-
Of the MPZ (P0) mutations that cause CMT1B, many affect adhesion of myelin lamellae, leading to unstable myelin. Other mutations probably have other kinds of gain-of-function effects.
-
Most GJB1 (Cx32) mutations cause a loss of function, probably by disrupting gap junction-mediated diffusion across the myelin sheath.
-
Transcription factors regulating the expression of myelin genes, including early growth response 2 (EGR2) and SOX10, are mutated in demyelinating forms of hereditary neuropathies.
-
Demyelination disrupts axon–Schwann cell interactions and has numerous effects on axons (for example, reduction of calibre, reorganization of ion channels, alteration of neurofilament density and phosphorylation) leading to deficiencies in axonal transport. Altered axonal transport can lead to distally accentuated axonal loss, which is responsible for the clinical disability of patients with inherited demyelinating neuropathies.
-
Mutations affecting components of the axonal cytoskeleton, including neurofilaments and the molecular motor KIF1Bβ, are mutated in axonal forms of CMT. Together with the findings that KIF5A mutations cause inherited spastic paraplegia, and a mutation of dynactin causes motor neuron disease, these data indicate that axonal transport is an important contributor to axonal atrophy and length-dependent axonal loss in these related disorders.
Abstract
Inherited neuropathies are caused by dominant or recessive mutations in genes that are expressed by neurons and/or Schwann cells. In demyelinating neuropathies, the deleterious effects originate primarily in myelinating Schwann cells. In axonal neuropathies, neurons (axons) are initially affected. In demyelinating neuropathies, the axonal cytoskeleton is altered and axonal transport is disrupted. In some axonal neuropathies, genes that are directly involved in axonal transport are mutated. So, a common consequence of inherited neuropathies is disruption of the ability of neurons to transport cargo efficiently along the entire length of their axons. These findings correlate with the observations that axonal atrophy and/or loss are primarily responsible for the clinical disability in hereditary neuropathies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lupski, J. R. & Garcia, C. A. in The Metabolic & Molecular Basis of Inherited Disease (eds Scriver, C. R. et al.) 5759–5788 (McGraw-Hill, New York, 2001).
Berger, P., Young, P. & Suter, U. Molecular cell biology of Charcot–Marie–Tooth disease. Neurogenetics 4, 1–15 (2002).
Kleopa, K. A. & Scherer, S. S. Inherited Neuropathies. Neurol. Clin. 20, 679–709 (2002).
Harding, A. E. & Thomas, P. K. The clinical features of hereditary motor and sensory neuropathy types I and II. Brain 103, 259–280 (1980).
Dyck, P. J., Chance, P., Lebo, R. & Carney, J. A. in Peripheral Neuropathy (eds Dyck, P. J. et al.) 1094–1136 (W. B. Saunders, Philadelphia, 1993).
Aguayo, A. J., Attiwell, M., Trecarten, J., Perkins, C. S. & Bray, C. M. Abnormal myelination in transplanted Trembler mouse Schwann cells. Nature 265, 73–75 (1977).
Chance, P. F. et al. DNA deletion associated with hereditary neuropathy with liability to pressure palsies. Cell 72, 143–151 (1993).
Windebank, T. in Peripheral Neuropathy (eds Dyck, P. J. et al.) 1137–1148 (W. B. Saunders, Philadelphia, 1993).
Adlkofer, K. et al. Heterozygous peripheral myelin protein 22-deficient mice are affected by a progressive demyelinating peripheral neuropathy. J. Neurosci. 17, 4662–4671 (1997).
Birouk, N. et al. Charcot–Marie–Tooth disease type 1A with 17p11.2 duplication. Clinical and electrophysiological phenotype study and factors influencing disease severity in 119 cases. Brain 120, 813–823 (1997).
Thomas, P. K. et al. The phenotypic manifestations of chromosome 17p11.2 duplication. Brain 120, 465–478 (1997).
Krajewski, K. M. et al. Neurological dysfunction and axonal degeneration in Charcot–Marie–Tooth disease. Brain 123, 1516–1527 (2000).
Fabrizi, G. M. et al. Clinical and pathological correlations in Charcot–Marie–Tooth neuropathy type 1A with the 17p11.2p12 duplication: a cross-sectional morphometric and immunohistochemical study in twenty cases. Muscle Nerve 21, 869–877 (1998).
Adlkofer, K. et al. Hypermyelination and demyelinating peripheral neuropathy in Pmp22-deficient mice. Nature Genet. 11, 274–280 (1995).
Vallat, J. M. et al. Ultrastructural PMP22 expression in inherited demyelinating neuropathies. Ann. Neurol. 39, 813–817 (1996).
Tobler, A. R. et al. Transport of Trembler-J mutant peripheral myelin protein 22 is blocked in the intermediate compartment and affects the transport of the wild-type protein by direct interaction. J. Neurosci. 19, 2027–2036 (1999).
D'Urso, D., Ehrhardt, P. & Müller, H. W. Peripheral myelin protein 22 and protein zero: a novel association in peripheral nervous system myelin. J. Neurosci. 19, 3396–3403 (1999).
Tobler, A. R., Liu, N., Mueller, L. & Shooter, E. M. Differential aggregation of the Trembler and Trembler J mutants of peripheral myelin protein 22. Proc. Natl Acad Sci. USA 99, 483–488 (2002).
Martini, R., Zielasek, J., Toyka, K. V., Giese, K. P. & Schachner, M. Protein zero (P0)-deficient mice show myelin degeneration in peripheral nerves characteristic of inherited human neuropathies. Nature Genet. 11, 281–285 (1995).
Sereda, M. et al. A transgenic rat model of Charcot–Marie–Tooth disease. Neuron 16, 1049–1060 (1996).
Chies, R. et al. Alterations in the Arf6-regulated plasma membrane endosomal recycling pathway in cells overexpressing the tetraspan protein Gas3/PMP22. J. Cell Sci. 116, 987–99 (2003).
Simons, M. et al. Overexpression of the myelin proteolipid protein leads to accumulation of cholesterol and proteolipid protein in endosomes/lysosomes: implications for Pelizaeus–Merzbacher disease. J. Cell Biol. 157, 327–336 (2002).
Hasse, B., Bosse, F. & Müller, H. W. Proteins of peripheral myelin are associated with glycosphingolipid/cholesterol-enriched membranes. J. Neurosci. Res. 69, 227–232 (2002).
Erne, B., Sansano, S., Frank, M. & Schaeren-Wiemers, N. Rafts in adult peripheral nerve myelin contain major structural myelin proteins and myelin and lymphocyte protein (MAL) and CD59 as specific markers. J. Neurochem. 82, 550–562 (2002).
Zoidl, G., Blass-Kampmann, S., D'Urso, D., Schmalenback, C. & Müller, H. W. Retroviral-mediated gene transfer of the peripheral myelin protein PMP22 in Schwann cells: modulation of cell growth. EMBO J. 14, 1122–1128 (1995).
Hanemann, C. O. & Müller, H. W. Pathogenesis of Charcot–Marie–Tooth IA (CMTIA) neuropathy. Trends Neurosci. 21, 282–286 (1998).
Fabbretti, E., Edomi, P., Brancolini, C. & Schneider, C. Apoptotic phenotype induced by overexpression of wild-type gas3/PMP22: its relation to the demyelinating peripheral neuropathy CMT1A. Genes Dev. 9, 1846–1856 (1995).
Brancolini, C. et al. Rho-dependent regulation of cell spreading by the tetraspan membrane protein Gas3/PMP22. Mol. Biol. Cell 10, 2441–2459 (1999).
Sancho, S., Young, P. & Suter, U. Regulation of Schwann cell proliferation and apoptosis in PMP22-deficient mice and mouse models of Charcot–Marie–Tooth disease type 1A. Brain 124, 2177–2187 (2001).
Wilson, H. L., Wilson, S. A., Surprenant, A. & North, R. A. Epithelial membrane proteins induce membrane blebbing and interact with the P2X7 receptor C terminus. J. Biol. Chem. 277, 34017–23 (2002).
Stevens, B. & Fields, R. D. Response of Schwann cells to action potentials in development. Science 287, 2267–2271 (2000).
Giese, K. P., Martini, R., Lemke, G., Soriano, P. & Schachner, M. Mouse P0 gene disruption leads to hypomyelination, abnormal expression of recognition molecules, and degeneration of myelin and axons. Cell 71, 565–576 (1992).
Montag, D. et al. Mice deficient for the myelin-associated glycoprotein show subtle abnormalities in myelin. Neuron 13, 229–246 (1994).
Niemann, S., Sereda, M. W., Suter, U., Griffiths, I. R. & Nave, K. A. Uncoupling of myelin assembly and Schwann cell differentiation by transgenic overexpression of peripheral myelin protein 22. J. Neurosci. 20, 4120–4128 (2000).
Taylor, V. & Suter, U. Epithelial membrane protein-2 and epithelial membrane protein-3: two novel members of the peripheral myelin protein 22 gene family. Gene 175, 115–120 (1996).
Wadehra, M., Iyer, R., Goodglick, L. & Braun, J. The tetraspan protein epithelial membrane protein-2 interacts with β1 integrins and regulates adhesion. J. Biol. Chem. 277, 41094–100 (2002).
Nave, K. -A. & Boespflug-Tanguy, O. Developmental defects of myelin formation: from X-linked mutations to human dysmyelinating diseases. Neuroscientist 2, 33–43 (1996).
Gudz, T. I., Schneider, T. E., Haas, T. A. & Macklin, W. B. Myelin proteolipid protein forms a complex with integrins and may participate in integrin receptor signaling in oligodendrocytes. J. Neurosci. 22, 7398–7407 (2002).
Feltri, M. L. et al. Conditional disruption of β1 integrin in Schwann cells impedes interactions with axons. J. Cell Biol. 156, 199–209 (2002). Conditional deletion of β1 integrin in Schwann cells causes a severe neuropathy with missorted axons.
Misko, A., Ferguson, T. & Notterpek, L. Matrix metalloproteinase mediated degradation of basement membrane proteins in Trembler J neuropathy nerves. J. Neurochem. 83, 885–894 (2002).
Adlkofer, K., Naef, R. & Suter, U. Analysis of compound heterozygous mice reveals that the Trembler mutation can behave as a gain-of-function allele. J. Neurosci. Res. 49, 671–680 (1997).
Naef, R., Adlkofer, K., Lescher, B. & Suter, U. Aberrant protein trafficking in Trembler suggests a disease mechanism for hereditary human peripheral neuropathies. Mol. Cell. Neurosci. 9, 13–25 (1997).
Naef, R. & Suter, U. Impaired intracellular trafficking is a common disease mechanism of PMP22 point mutations in peripheral neuropathies. Neurobiol. Dis. 6, 1–14 (1999).
D'Urso, D., Schmalenbach, C., Zoidl, G., Prior, R. & Müller, H. W. Studies on the effects of altered PMP22 expression during myelination in vitro. J. Neurosci. Res. 48, 31–42 (1997).
Colby, J. et al. PMP22 carrying the Trembler or Trembler-J mutation is intracellularly retained in myelinating Schwann cells. Neurobiol. Dis. 7, 561–573 (2000).
Dickson, K. M. et al. Association of calnexin with mutant peripheral myelin protein-22 ex vivo: A basis for 'gain-of-function' ER diseases. Proc. Natl Acad. Sci. USA 99, 9852–9857 (2002). PMP22 mutants have a prolonged association with the endoplasmic reticulum chaperone protein calnexin, possibly depleting the pool of calnexin and causing neuropathy.
Isaacs, A. M. et al. Identification of a new Pmp22 mouse mutant and trafficking analysis of a Pmp22 allelic series suggesting that protein aggregates may be protective in Pmp22-associated peripheral neuropathy. Mol. Cell. Neurosci. 21, 114–125 (2002).
Pareek, S. et al. Neurons promote the translocation of peripheral myelin protein 22 into myelin. J. Neurosci. 17, 7754–7762 (1997).
Southwood, C. M., Garbern, J., Jiang, W. & Gow, A. The unfolded protein response modulates disease severity in Pelizaeus–Merzbacher disease. Neuron 36, 585–596 (2002). Mutants in PLP induce an 'unfolded protein response' in oligodendrocytes; in contrast to most situations, this seems to be adaptive. This finding might be relevant to disease mechanisms in neuropathies.
Denzel, A. et al. Early postnatal death and motor disorders in mice congenitally deficient in calnexin expression. Mol. Cell. Biol. 22, 7398–404 (2002).
Notterpek, L., Shooter, E. M. & Snipes, G. J. Upregulation of the endosomal-lysosomal pathway in the Trembler-J neuropathy. J. Neurosci. 17, 4190–4200 (1997).
Notterpek, L., Ryan, M. C., Tobler, A. R. & Shooter, E. M. PMP22 accumulation in aggresomes: implications for CMT1A pathology. Neurobiol. Dis. 6, 450–460 (1999).
Ryan, M. C., Shooter, E. M. & Notterpek, L. Aggresome formation in neuropathy models based on peripheral myelin protein 22 mutations. Neurobiol. Dis. 10, 109–118 (2002).
Gamp, A. C. et al. LIMP-2/LGP85 deficiency causes ureteric pelvic junction obstruction, deafness and peripheral neuropathy in mice. Hum. Mol. Genet. 12, 631–46 (2003).
Lemke, G. & Axel, R. Isolation and sequence of a cDNA encoding the major structural protein of peripheral myelin. Cell 40, 501–508 (1985).
Shapiro, L., Doyle, J. P., Hensley, P., Colman, D. R. & Hendrickson, W. A. Crystal stucture of the extracellular domain from P0, the major structural protein of peripheral nerve myelin. Neuron 17, 435–449 (1996).
Martini, R., Mohajeri, M. H., Kasper, S., Giese, K. P. & Schachner, M. Mice doubly deficient in the genes for P0 and myelin basic protein show that both proteins contribute to the formation of the major dense line in peripheral nerve myelin. J. Neurosci. 15, 4488–4495 (1995).
D'Urso, D. et al. Protein zero of peripheral nerve myelin: biosynthesis, membrane insertion, and evidence for homotypic interaction. Neuron 4, 449–460 (1990).
Filbin, M. T., Walsh, F. S., Trapp, B. D., Pizzey, J. A. & Tennekoon, G. I. Role of P0 protein as a homophilic adhesion molecule. Nature 344, 871–872 (1990).
Xu, W. B. et al. Mutations in the cytoplasmic domain of P0 reveal a role for PKC-mediated phosphorylation in adhesion and myelination. J. Cell Biol. 155, 439–445 (2001). A CMT1B mutation that abolishes the protein kinase C phosphorylation of P0 causes loss of P0-mediated adhesion, providing an important clue towards signal transduction mediated by P0.
Wrabetz, L. et al. P0 glycoprotein overexpression causes congenital hypomyelination of peripheral nerves. J. Cell Biol. 148, 1021–1033 (2000).
Yin, X. et al. Schwann cell myelination requires timely and precise targeting of P0 protein. J. Cell Biol. 148, 1009–1020 (2000).
Previtali, S. C. et al. Epitope-tagged P0 glycoprotein causes Charcot—Marie–Tooth-like neuropathy in transgenic mice. J. Cell Biol. 151, 1035–1045 (2000).
Willecke, K. et al. Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 383, 725–737 (2002).
White, T. W. & Paul, D. L. Genetic diseases and gene knockouts reveal diverse connexin functions. Annu. Rev. Physiol. 61, 283–310 (1999).
Bergoffen, J. et al. Connexin mutations in X-linked Charcot–Marie–Tooth disease. Science 262, 2039–2042 (1993).
Anzini, P. et al. Structural abnormalities and deficient maintenance of peripheral nerve myelin in mice lacking the gap junction protein connexin32. J. Neurosci. 17, 4545–4561 (1997).
Scherer, S. S. et al. Connexin32-null mice develop a demyelinating peripheral neuropathy. Glia 24, 8–20 (1998).
Balice-Gordon, R. J., Bone, L. J. & Scherer, S. S. Functional gap junctions in the Schwann cell myelin sheath. J. Cell Biol. 142, 1095–1104 (1998). Dye transfer studies demonstrate that incisures contain 'reflexive' gap junctions that provide a radial pathway for diffusion of small molecules across the myelin sheath. The correct functioning of this system might be crucial for maintaining myelin sheaths.
Altevogt, B. M., Kleopa, K. A., Postma, F. R., Scherer, S. S. & Paul, D. L. Cx29 is uniquely distributed within myelinating glial cells of the central and peripheral nervous systems. J. Neurosci. 22, 6458–6470 (2002).
Li, X. et al. Connexin29 expression, immunocytochemistry and freeze-fracture replica immunogold labelling (FRIL) in sciatic nerve. Eur. J. Neurosci. 16, 795–806 (2002).
Lewis, R. A., Sumner, A. J. & Shy, M. E. Electrophysiological features of inherited demyelinating neuropathies: a reappraisal in the era of molecular diagnosis. Muscle Nerve 23, 1472–1487 (2000).
Hahn, A., Ainsworth, P. J., Bolton, C. F., Bilbao, J. M. & Vallat, J. -M. Pathological findings in the X-linked form of Charcot–Marie–Tooth disease: a morphometric and ultrastructural analysis. Acta Neuropathol. 101, 129–139 (2001).
Odermatt, B. et al. Connexin 47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS. J. Neurosci. 23, 4549–4559 (2003).
Menichella, D. M., Goodenough, D. A., Sirkowski, E., Scherer, S. S. & Paul, D. L. Connexins are critical for normal myelination in the central nervous system. J. Neurosci. 23, 5963–5973 (2003).
Kleopa, K. A., Yum, S. W. & Scherer, S. S. Cellular mechanisms of connexin32 mutations associated with CNS manifestations. J. Neurosci. Res. 68, 522–534 (2002).
Yum, S. W., Kleopa, K. A., Shumas, S. & Scherer, S. S. Diverse trafficking abnormalities for connexin32 mutants causing CMTX. Neurobiol. Dis. 11, 43–52 (2002).
Bruzzone, R., T. W. White, S. S. Scherer, Fischbeck, K. H. & Paul, D. L. Null mutations of connexin32 in patients with X-linked Charcot–Marie–Tooth disease. Neuron 13, 1253–1260 (1994).
Rouan, F. et al. Trans-dominant inhibition of connexin-43 by mutant connexin-26: implications for dominant connexin disorders affecting epidermal differentiation. J. Cell Sci. 114, 2105–2113 (2001).
VanSlyke, J. K., Deschênes, S. M. & Musil, L. S. Intracellular transport, assembly, and degradation of wild-type and disease-linked mutant gap junction proteins. Mol. Biol. Cell 11, 1933–1946 (2000).
Abrams, C. K., Oh, S., Ri, Y. & Bargiello, T. A. Mutations in connexin 32: the molecular and biophysical bases for the X-linked form of Charcot–Marie–Tooth disease. Brain Res. Rev. 32, 203–214 (2000).
Castro, C., Gomez-Hernandez, J. M., Silander, K. & Barrio, L. C. Altered formation of hemichannels and gap junction channels caused by C-terminal connexin-32 mutations. J. Neurosci. 19, 3752–3760 (1999).
Tang, X., Fenton, M. J. & Amar, S. Identification and functional characterization of a novel binding site on TNF-α promoter. Proc. Natl Acad. Sci. USA 100, 4096–4101 (2003).
Moriwaki, Y. et al. Mycobacterium bovis bacillus Calmette-Guerin and its cell wall complex induce a novel lysosomal membrane protein, SIMPLE, that bridges the missing link between lipopolysaccharide and p53-inducible gene, LITAF (PIG7) and estrogen-inducible gene, EET-1. J. Biol. Chem. 276, 23065–23076 (2001).
Liu, H., Nakagawa, T., Kanematsu, T., Uchida, T. & Tsuji, S. Isolation of 10 differentially expressed cDNAs in differentiated Neuro2a cells induced through controlled expression of the GD3 synthase gene. J. Neurochem. 72, 1781–1790 (1999).
Cuesta, A. et al. The gene encoding ganglioside-induced differentiation-associated protein 1 is mutated in axonal Charcot–Marie–Tooth type 4A disease. Nature Genet. 30, 22–25 (2002).
Baxter, R. V. et al. Ganglioside-induced differentiation-associated protein-1 is mutant in Charcot–Marie–Tooth disease type 4A/8q21. Nature Genet. 30, 21–22 (2002).
Berger, P., Bonneick, S., Willi, S., Wymann, M. & Suter, U. Loss of phosphatase activity in myotubularin-related protein 2 is associated with Charcot–Marie–Tooth disease type 4B1. Hum. Mol. Genet. 11, 1569–1579 (2002).
Bolino, A. et al. Molecular characterization and expression analysis of Mtmr2, a mouse homologue of MTMR2, the myotubularin-related-2 gene mutated in CMT4B. Gene 283, 17–26 (2002).
Laporte, J., Blondeau, F., Buj-Bello, A. & Mandel, J. L. The myotubularin family: from genetic disease to phosphoinositide metabolism. Trends Genet. 17, 221–228 (2001).
Wishart, M. J. & Dixon, J. E. PTEN and myotubularin phosphatases: from 3-phosphoinositide dephosphorylation to disease. Phosphatase and tensin homolog deleted on chromosome ten. Trends Cell Biol. 12, 579–585 (2002).
Schaletzky, J. et al. Phosphatidylinositol-5-phosphate activation and conserved substrate specificity of the myotubularin phosphatidylinositol 3-phosphatases. Curr. Biol. 13, 504–509 (2003).
Kim, S. A., Vacratsis, P. O., Firestein, R., Cleary, M. L. & Dixon, J. E. Regulation of myotubularin-related (MTMR) 2 phosphatidylinositol phosphatase by MTMR5, a catalytically inactive phosphatase. Proc. Natl Acad. Sci. USA 100, 4492–4497 (2003).
Nandukar, H. H. et al. Identification of myotubularin as the lipid phosphatase catalytic subunit associated with the 3-phosphatase adapter protein, 3-PAP. Proc. Natl Acad. Sci. USA 100, 8660–8665 (2003).
Kalaydjieva, L. et al. N-myc downstream-regulated gene 1 is mutated in hereditary motor and sensory neuropathy-Lom. Am. J. Hum. Genet. 67, 47–58 (2000).
Gillespie, C. S. et al. Peripheral demyelination and neuropathic pain behavior in periaxin-deficient mice. Neuron 26, 523–531 (2000). Prx−/− mice develop a demyelinating neuropathy with enhanced sensitivity to pain; these findings mirror those in PRX -null patients.
Gillespie, C. S., Sherman, D. L., Blair, G. E. & Brophy, P. J. Periaxin, a novel protein of myelinating Schwann cells with a possible role in axonal ensheathment. Neuron 12, 497–508 (1994).
Scherer, S. S., Xu, Y. -T., Bannerman, P., Sherman, D. L. & Brophy, P. J. Periaxin expression in myelinating Schwann cells: modulation by axon-glial interactions and polarized localization during development. Development 121, 4265–4273 (1995).
Sherman, D. L., Fabrizi, C., Gillespie, C. S. & Brophy, P. J. Specific disruption of a Schwann cell dystrophin-related protein complex in a demyelinating neuropathy. Neuron 30, 677–687 (2001).
Rambukkana, A., Zanazzi, G., Tapinos, N. & Salzer, J. L. Contact-dependent demyelination by Mycobacterium leprae in the absence of immune cells. Science 296, 927–931 (2002).
Topilko, P. et al. Krox-20 controls myelination in the peripheral nervous system. Nature 371, 796–799 (1994).
Warner, L. E. et al. Mutations in the early growth response 2 (EGR2) gene are associated with hereditary myelinopathies. Nature Genet. 18, 382–384 (1998).
Nagarajan, R. et al. EGR2 mutations in inherited neuropathies dominant-negatively inhibit myelin gene expression. Neuron 30, 355–368 (2001). EGR2 mutants that cause demyelinating neuropathies reduce the expression of myelin-related genes by wild-type EGR2 in a dominant manner.
Zorick, T. S., Syroid, D. E., Brown, A., Gridley, T. & Lemke, G. Krox-20 controls SCIP expression, cell cycle exit and susceptibility to apoptosis in developing myelinating Schwann cells. Development 126, 1397–1406 (1999).
Musso, M., Balestra, P., Taroni, F., Bellone, E. & Mandich, P. Different consequences of EGR2 mutants on the transactivation of human cx32 promoter. Neurobiol. Dis. 12, 89–95 (2003).
Warner, L. E., Svaren, J., Milbrandt, J. & Lupski, J. R. Functional consequences of mutations in the early growth response 2 gene (EGR2) correlate with severity of human myelinopathies. Hum. Mol. Genet. 8, 1245–1251 (1999).
Peirano, R. I., Goerich, D. E., Riethmacher, D. & Wegner, M. Protein zero gene expression is regulated by the glial transcription factor Sox10. Mol. Cell. Biol. 20, 3198–3209 (2000).
Bondurand, N. et al. Human connexin 32, a gap junction protein altered in the X-linked form of Charcot–Marie–Tooth disease, is directly regulated by the transcription factor SOX10. Hum. Mol. Genet. 10, 2783–2795 (2001). A CMTX-associated mutation in the Cx32 promoter abolishes its normal activation by SOX10. This links the two proteins in a common functional pathway that is disrupted in some neuropathies.
Paratore, C., Eichenberger, C., Suter, U. & Sommer, L. Sox10 haploinsufficiency affects maintenance of progenitor cells in a mouse model of Hirschsprung disease. Hum. Mol. Genet. 11, 3075–3085 (2002).
Kim, J., Lo, L., Dormand, E. & Anderson, D. J. SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron 38, 17–31 (2003).
Stolt, C. C. et al. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev. 16, 165–170 (2002).
Britsch, S. et al. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 15, 66–78 (2001).
Zhao, C. et al. Charcot–Marie–Tooth disease type 2A caused by mutation in a microtubule motor KIF1Bβ. Cell 105, 587–597 (2001).
Hirokawa, N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 279, 519–526 (1998).
Mok, H. et al. Association of the kinesin superfamily motor protein KIF1Bα with postsynaptic density-95 (PSD-95), synapse-associated protein-97, and synaptic scaffolding molecule PSD-95/discs large/zona occludens-1 proteins. J. Neurosci. 22, 5253–5258 (2002).
Griffin, J. W. & Watson, D. F. Axonal transport in neurologic disease. Ann. Neurol. 23, 3–13 (1988).
Zhu, Q., Couillard-Despres, S. & Julien, J. P. Delayed maturation of regenerating myelinated axons in mice lacking neurofilaments. Exp. Neurol. 148, 299–316 (1997).
Lee, M. K., Marzalek, J. R. & Cleveland, D. W. A mutant neurofilament subunit causes massive, selective motor neuron death: implications for the pathogenesis of human motor neuron disease. Neuron 13, 975–988 (1994).
Ohara, O., Gahara, Y., Miyake, T., Teraoka, H. & Kitamura, T. Neurofilament deficiency in quail caused by nonsense mutation in neurofilament-L gene. J. Cell Biol. 121, 387–395 (1993).
Brownlees, J. et al. Charcot–Marie–Tooth disease neurofilament mutations disrupt neurofilament assembly and axonal transport. Hum. Mol. Genet. 11, 2837–2844 (2002).
Perez-Olle, R., Leung, C. L. & Liem, R. K. H. Effects of Charcot–Marie–Tooth-linked mutations of the neurofilament light subunit on intermediate filament formation. J. Cell Sci. 115, 4937–4946 (2002). Together, references 120 and 121 show that mutant NEFL proteins do not properly form intermediate filaments, have dominant effects on the assembly of wild-type neurofilaments and disrupt the axonal transport of neurofilaments.
Al-Chalabi, A. & Miller, C. C. Neurofilaments and neurological disease. Bioessays 25, 346–355 (2003).
Crosby, A. H. & Proukakis, C. Is the transportation highway the right road for hereditary spastic paraplegia? Am. J. Hum. Genet. 71, 1009–1016 (2002).
Reid, E. et al. A kinesin heavy chain (KIF5A) mutation in hereditary spastic paraplegia (SPG10). Am. J. Hum. Genet. 71, 1189–1194 (2002).
Xia, C. H. et al. Abnormal neurofilament transport caused by targeted disruption of neuronal kinesin heavy chain KIF5A. J. Cell Biol. 161, 55–66 (2003). Mice lacking neuronal KIF5A have greatly diminished transport of neurofilaments.
LaMonte, B. H. et al. Disruption of dynein/dynactin inhibits axonal transport in motor neurons causing late-onset porgressive degeneration. Neuron 34, 715–727 (2002).
Puls, I. et al. Mutant dynactin in motor neuron disease. Nature Genet. 33, 455–456 (2003). A mutation in the p150 dynactin subunit causes motor neuron disease; the corresponding mutant protein has decreased binding to microtubules.
Bomont, P. et al. The gene encoding gigaxonin, a new member of the cytoskeletal BTB/kelch repeat family, is mutated in giant axonal neuropathy. Nature Genet. 26, 370–374 (2000).
Ding, J. Q. et al. Microtubule-associated protein 1B: a neuronal binding partner for gigaxonin. J. Cell Biol. 158, 427–433 (2002).
Auer-Grumbach, M. et al. Autosomal dominant inherited neuropathies with prominent sensory loss and mutilations: a review. Arch. Neurol. 60, 329–334 (2003).
Echard, A. et al. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science 279, 580–585 (1998).
Cantalupo, G., Alifano, P., Roberti, V., Bruni, C. B. & Bucci, C. Rab-interacting lysosomal protein (RILP): the Rab7 effector required for transport to lysosomes. EMBO J. 20, 683–693 (2001).
Jordens, I. et al. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein–dynactin motors. Curr. Biol. 11, 1680–1685 (2001).
Verhoeven, K. et al. Mutations in the small GTP-ase late endosomal protein RAB7 cause Charcot–Marie–Tooth disease type 2B neuropathy. Am. J. Hum. Genet. 72 (2003).
Choudhury, A. et al. Rab proteins mediate Golgi transport of caveola-internalized glycosphingolipids and correct lipid trafficking in Niemann–Pick C cells. J. Clin. Invest. 109, 1541–1550 (2002).
Ostlund, C. & Worman, H. J. Nuclear envelope proteins and neuromuscular diseases. Muscle Nerve 27, 393–406 (2003).
Eriksson, M. et al. Recurrent de novo point mutations in lamin A cause Hutchinson–Gilford progeria syndrome. Nature 429, 293–298 (2003).
Sullivan, T. et al. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J. Cell Biol. 147, 913–920 (1999).
Antonellis, A. et al. Glycyl tRNA synthetase mutations in Charcot–Marie–Tooth disease type 2D and distal spinal muscular atrophy type V. Am. J. Hum. Genet. 72, 1293–1299 (2003).
Freist, W., Logan, D. T. & Gauss, D. H. Glycyl-tRNA synthetase. Biol. Chem. Hoppe Seyler 377, 343–56 (1996).
Martini, R. The effect of myelinating Schwann cells on axons. Muscle Nerve 24, 456–466 (2001).
Peles, E. & Salzer, J. L. Molecular domains of myelinated fibers. Curr. Opin. Neurobiol. 10, 558–565 (2000).
Lappe-Siefke, C. et al. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nature Genet. 33, 366–374 (2003). Axonal pathologies are the only defect in mice lacking the myelin-related protein CNP, which is not expressed by neurons. This indicates that secondary axonal damage can occur without morphologically detectable effects in myelinating glia.
Mäurer, M. et al. Role of immune cells in animal models for inherited neuropathies: facts and visions. J. Anat. 200, 405–414 (2002).
Sancho, S., Magyar, J. P., Aguzzi, A. & Suter, U. Distal axonopathy in peripheral nerves of PMP22 mutant mice. Brain 122, 1563–1577 (1999). The number of large, myelinated axons is reduced in a length-dependent pattern in Pmp22 mutant mice, demonstrating axonopathy in a genetically authentic animal model of a demyelinating neuropathy.
Perea, J. et al. Induced myelination and demyelination in a conditional mouse model of Charcot–Marie–Tooth disease type 1A. Hum. Mol. Genet. 10, 1007–1018 (2001). Demyelination in adult mice can be induced by overexpressing Pmp22 in myelinating Schwann cells. Conversely, normalizing Pmp22 expression in diseased Schwann cells increases the degree of remyelination, indicating that some of the defects are reversible.
Samsam, M. et al. The WldS mutation delays robust loss of motor and sensory axons in a genetic model for myelin-related axonopathy. J. Neurosci. 23, 2833–2839 (2003). The neuronal expression of a chimeric protein that delays Wallerian degeneration delays axonal loss in Mpz−/− mice. This provides proof-of-principle that axonal loss can be ameliorated in an animal model.
Acknowledgements
We apologize to our colleagues whose work we could not cite owing to space restrictions. In particular, please consult the Mutation Database for Inherited Peripheral Neuropathies for the original references referring to the different mutations found in hereditary neuropathies. The work in our laboratories is supported by the Swiss National Science Foundation, the National Center of Competence in Research 'Neural Plasticity and Repair' (to U.S.), the Charcot–Marie–Tooth Association, the National Multiple Sclerosis Society and the National Institutes of Health (to S.S.S.).
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
LocusLink
OMIM
Waardenburg–Shah/Waardenburg type IV syndrome
FURTHER INFORMATION
Charcot–Marie–Tooth Association
Mutation Database for Inherited Peripheral Neuropathies
Muscular Dystrophy Association
Glossary
- ONION BULB
-
A concentric arrangement of supernumerary Schwann cells around an incompletely remyelinated axon, which is thought to represent repeated cycles of demyelination and remyelination.
- HYPOMYELINATED AXON
-
A remyelinated axon with a myelin sheath that is inappropriately thin for the axonal calibre.
- RAFTS
-
Domains of the plasma membrane enriched in sphingolipids and cholesterol. They incorporate lipid-conjugated proteins and therefore serve to assemble proteins involved in signal transduction.
- TETRASPAN
-
Proteins with four membrane-spanning domains.
- INTEGRINS
-
A large family of transmembrane proteins that act mainly as receptors for extracellular matrix molecules.
- DOMINANT-NEGATIVE
-
A mutant molecule that can form a heteromeric complex with the normal molecule, reducing the activity of the entire complex.
- EPITOPE-TAG
-
The immunological determinant of an antigen that has been fused to a protein of interest for its subsequent localization with specific antibodies.
- CALNEXIN
-
A calcium-binding protein of the endoplasmic reticulum that processes and monitors endoplasmic reticulum proteins, retaining those that are unassembled or incorrectly folded.
- CMT2-LIKE PHENOTYPE
-
A late-onset neuropathy with pronounced axonal loss.
- GAP JUNCTION
-
A junction between two cells consisting of pores that allow passage of molecules (up to 1 kDa).
- PLECKSTRIN HOMOLOGY DOMAIN
-
A sequence of about 100 amino acids that is present in many signalling molecules. Pleckstrin is a protein of unknown function that was originally identified in platelets. It is a principal substrate of protein kinase C.
- COILED-COIL DOMAIN
-
A protein domain that forms a bundle of two or three α-helices. Whereas short coiled-coil domains are involved in protein interactions, long coiled-coil domains, which form long rods, occur in structural or motor proteins.
- PDZ-BINDING MOTIF
-
A peptide-binding domain that is important for the organization of membrane proteins, particularly at cell–cell junctions, including synapses. PDZ-domain-containing proteins bind to the PDZ-binding motifs that are located at the carboxyl termini of proteins or can form dimers with other PDZ domains. PDZ domains are named after the proteins in which these sequence motifs were originally identified (PSD95, Discs large, zona occludens 1).
- GLAUCOMA
-
A group of eye diseases characterized by an increase in intraocular pressure which causes pathological changes in the optic disk and typical defects in visual fields.
- HAPLOINSUFFICIENCY
-
Loss of one copy (one allele) of a gene is sufficient to give rise to disease. Haploinsufficiency implies that no dominant-negative effect of the mutated gene product has to be invoked.
- AMYOTROPHIC LATERAL SCLEROSIS
-
A progressive neurological disease that is associated with the degeneration of central and spinal motor neurons. This neuron loss causes muscles to weaken and atrophy.
- DYNEIN–DYNACTIN
-
Dynein is a motor protein complex involved in minus end-directed microtubule transport. Dynactin is a biochemically separable complex that links dynein to target organelles.
Rights and permissions
About this article
Cite this article
Suter, U., Scherer, S. Disease mechanisms in inherited neuropathies. Nat Rev Neurosci 4, 714–726 (2003). https://doi.org/10.1038/nrn1196
Issue Date:
DOI: https://doi.org/10.1038/nrn1196
This article is cited by
-
Expanding the phenotypic spectrum of Dejerine-Sottas syndrome caused by the trembler mutation
neurogenetics (2022)
-
Myelin Biology
Neurotherapeutics (2021)
-
Electron Microscopy Analysis of Sciatic Nerve Fibers in C57BL/6 Transgenic Mice
Neurophysiology (2020)
-
A histone deacetylase 3–dependent pathway delimits peripheral myelin growth and functional regeneration
Nature Medicine (2018)
-
Glutathione-conjugating and membrane-remodeling activity of GDAP1 relies on amphipathic C-terminal domain
Scientific Reports (2016)