Key Points
-
Some of the clearest evidence on the localization of memory within the mammalian brain has come from studies of associative learning, especially through the use of classical or Pavlovian conditioning, in which animals learn to express a conditioned response to a predictive or conditioned stimulus (CS) paired with an unconditioned stimulus (US). This review focuses on two of the most widely used forms of Pavlovian learning: eyelid and fear conditioning.
-
In eyelid conditioning, the deep cerebellar nuclei and overlying cerebellar cortex seem to mediate the acquisition and storage of discrete motor memories. By contrast, evidence from fear conditioning points to the lateral nucleus of the amygdala (LA) as a key component of the brain system that is responsible for the formation of aversive emotional memories.
-
Despite being mediated by different brain systems, these forms of learning might use a similar sequence of events to form new memories. Recent data point to a 'trigger-and-storage' model, in which the initial encoding and subsequent long-term storage of memory are mediated by separate groups of neurons.
-
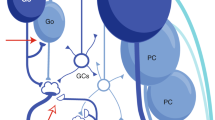
Two sets of cerebellar neurons, the Purkinje cells and neurons in the anterior interpositus nucleus, change their activity during eyelid conditioning and so represent potential sites of plasticity. Recent evidence indicates that the cerebellar cortex might participate in the initiation of learning, whereas the deep cerebellar nuclei are more involved in the long-term storage of memory.
-
The dorsal region of the LA (LAd) is a site of CS–US convergence. Two distinct sets of neurons in the LAd could contribute differentially to the initiation and storage of fear-conditioning memories. Cells in the dorsal tip of the LAd develop conditioning-induced enhancement of their auditory responses very early in training, but cells in more ventral regions take longer to reach maximal response levels.
-
Several important questions remain unanswered, including whether there is complete transfer of memory from the 'soft' trigger cells to the 'hard' storage cells. It is also not known whether plasticity in the trigger cells is required for the induction of plasticity in the storage cells, or if the induction of soft and hard plasticity can proceed independently, but at different rates.
Abstract
Recent evidence from cerebellum-dependent motor learning and amygdala-dependent fear conditioning indicates that, despite being mediated by different brain systems, these forms of learning might use a similar sequence of events to form new memories. In each case, learning seems to induce changes in two different groups of neurons. Changes in the first class of cells are induced very rapidly during the initial stages of learning, whereas changes in the second class of cells develop more slowly and are resistant to extinction. So, anatomically distinct cell populations might contribute differentially to the initial encoding and the long-term storage of memory in these two systems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Pavlov, I. P. Conditioned Reflexes. An Investigation of the Physiological Activity of the Cerebral Cortex (Oxford Univ. Press, London, 1927).
Gormezano, I., Prokasy, W. F. & Thompson, R. F. Classical Conditioning (Lawrence Erlbaum Associates, Hillsdale, New Jersey, 1987).
Gormezano, I., Kehoe, E. J. & Marshall, B. S. Twenty years of classical conditioning research with the rabbit. Prog. Psychobiol. Physiol. Psychol. 10, 197–275 (1983).
Schneiderman, N., Fuentes, I. & Gormezano, I. Acquisition and extinction of the classically conditioned eyelid response in the albino rabbit. Science 136, 650–652 (1962).
Mauk, M. D. & Ruiz, B. P. Learning-dependent timing of Pavlovian eyelid responses: differential conditioning using multiple interstimulus intervals. Behav. Neurosci. 106, 666–681 (1992).
Millenson, J. R., Kehoe, E. J. & Gormezano, I. Classical conditioning of the rabbit's nictitating membrane response under fixed and mixed CS–US intervals. Learn. Motiv. 8, 351–366 (1977).
Schreurs, B. G. Long-term memory and extinction of the classically conditioned rabbit nictitating membrane response. Learn. Motiv. 24, 293–302 (1993).
Medina, J. F., Garcia, K. S. & Mauk, M. D. A mechanism for savings in the cerebellum. J. Neurosci. 21, 4081–4089 (2001).Showed that plasticity located outside the cerebellar cortex persists after behavioural extinction. The amount of plasticity that remained after extinction was a reliable predictor of the rate of reacquisition.
Napier, R. M., Macrae, M. & Kehoe, E. J. Rapid reacquisition in conditioning of the rabbit's nictitating membrane response. J. Exp. Psychol. Anim. Behav. Process. 18, 182–192 (1992).
Lennartz, R. C. & Weinberger, N. M. Analysis of response systems in Pavlovian conditioning reveals rapidly versus slowly acquired conditioned responses: support for two factors, implications for behavior and neurobiology. Psychobiology 20, 93–119 (1992).
LeDoux, J. E. Emotion circuits in the brain. Annu. Rev. Neurosci. 23, 155–184 (2000).
Brown, J. S., Kalish, H. I. & Farber, I. E. Conditioned fear as revealed by magnitude of startle response to an auditory stimulus. J. Exp. Psychol. 41, 317–328 (1951).
Bolles, R. C. & Fanselow, M. S. A perceptual–defensive–recuperative model of fear and pain. Behav. Brain Sci. 3, 291–323 (1980).
Bouton, M. E. Conditioning, remembering, and forgetting. J. Exp. Psychol. Anim. Behav. Process. 20, 219–231 (1994).
Kim, J. J. & Thompson, R. F. Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends Neurosci. 20, 177–181 (1997).
Raymond, J. L., Lisberger, S. G. & Mauk, M. D. The cerebellum: a neuronal learning machine? Science 272, 1126–1131 (1996).
Fanselow, M. S. & LeDoux, J. E. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron 23, 229–232 (1999).
Blair, H. T., Schafe, G. E., Bauer, E. P., Rodrigues, S. M. & LeDoux, J. E. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learn. Mem. 8, 229–242 (2001).
Maren, S. Neurobiology of Pavlovian fear conditioning. Annu. Rev. Neurosci. 24, 897–931 (2001).
Aitkin, L. M. & Boyd, J. Acoustic input to the lateral pontine nuclei. Hear. Res. 1, 67–77 (1978).
Eccles, J. C., Ito, M. & Szentágothai, J. The Cerebellum as a Neuronal Machine (Springer, New York, 1967).
Llinas, R. in Handbook of Physiology II. The Nervous System (ed. Brokks, V. B.) 831–976 (Am. Physiol. Soc., Bethesda, Maryland, 1981).
Voogd, J. & Glickstein, M. The anatomy of the cerebellum. Trends Neurosci. 21, 370–375 (1998).
Solomon, P. R., Lewis, J. L., LoTurco, J. J., Steinmetz, J. E. & Thompson, R. F. The role of the middle cerebellar peduncle in acquisition and retention of the rabbit's classically conditioned nictitating membrane response. Bull. Psychon. Soc. 24, 74–78 (1986).
Lewis, J. L., LoTurco, J. J. & Solomon, P. R. Lesions of the middle cerebellar peduncle disrupt acquisition and retention of the rabbit's classically conditioned nictitating membrane response. Behav. Neurosci. 101, 151–157 (1987).
Steinmetz, J. E. et al. Classical conditioning of the rabbit eyelid response with a mossy-fiber stimulation CS. I. Pontine nuclei and middle cerebellar peduncle stimulation. Behav. Neurosci. 100, 878–887 (1986).
Hesslow, G., Svensson, P. & Ivarsson, M. Learned movements elicited by direct stimulation of cerebellar mossy fiber afferents. Neuron 24, 179–185 (1999).
Sears, L. L. & Steinmetz, J. E. Dorsal accessory inferior olive activity diminishes during acquisition of the rabbit classically conditioned eyelid response. Brain Res. 545, 114–122 (1991).
Linden, D. J. & Connor, J. A. Cellular mechanisms of long-term depression in the cerebellum. Curr. Opin. Neurobiol. 3, 401–406 (1993).
Ito, M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol. Rev. 81, 1143–1195 (2001).
McCormick, D. A., Steinmetz, J. E. & Thompson, R. F. Lesions of the inferior olivary complex cause extinction of the classically conditioned eyeblink response. Brain Res. 359, 120–130 (1985).
Yeo, C. H., Hardiman, M. J. & Glickstein, M. Classical conditioning of the nictitating membrane response of the rabbit. IV. Lesions of the inferior olive. Exp. Brain Res. 63, 81–92 (1986).
Welsh, J. P. & Harvey, J. A. Acute inactivation of the inferior olive blocks associative learning. Eur. J. Neurosci. 10, 3321–3332 (1998).
Mauk, M. D., Steinmetz, J. E. & Thompson, R. F. Classical conditioning using stimulation of the inferior olive as the unconditioned stimulus. Proc. Natl Acad. Sci. USA 83, 5349–5353 (1986).
Hesslow, G. & Ivarsson, M. Suppression of cerebellar Purkinje cells during conditioned responses in ferrets. Neuroreport 5, 649–652 (1994).
McCormick, D. A. & Thompson, R. F. Neuronal responses of the rabbit cerebellum during acquisition and performance of a classically conditioned nictitating membrane-eyelid response. J. Neurosci. 4, 2811–2822 (1984).The first clear evidence that the cerebellum is involved in eyelid conditioning. Lesions of the cerebellum prevented learning; recording data showed cells within the interpositus nucleus that developed activity resembling the conditioning response; stimulation of those interpositus sites caused eyelid closure.
Berthier, N. E. & Moore, J. W. Activity of deep cerebellar nuclear cells during classical conditioning of nictitating membrane extension in rabbits. Exp. Brain Res. 83, 44–54 (1990).
Berthier, N. E. & Moore, J. W. Cerebellar Purkinje cell activity related to the classically conditioned nictitating membrane response. Exp. Brain Res. 63, 341–350 (1986).
Gould, T. J., Sears, L. L. & Steinmetz, J. E. Possible CS and US pathways for rabbit classical eyelid conditioning: electrophysiological evidence for projections from the pontine nuclei and inferior olive to cerebellar cortex and nuclei. Behav. Neural Biol. 60, 172–185 (1993).
Steinmetz, J. E., Lavond, D. G. & Thompson, R. F. Classical conditioning in rabbits using pontine nucleus stimulation as a conditioned stimulus and inferior olive stimulation as an unconditioned stimulus. Synapse 3, 225–233 (1989).
Middleton, F. A. & Strick, P. L. Cerebellar output channels. Int. Rev. Neurobiol. 41, 61–82 (1997).
Ito, M. The Cerebellum and Neural Control (Raven, New York, 1984).
McCormick, D. A. & Thompson, R. F. Cerebellum: essential involvement in the classically conditioned eyelid response. Science 223, 296–299 (1984).
Shi, C. & Davis, M. Visual pathways involved in fear conditioning measured with fear-potentiated startle: behavioral and anatomic studies. J. Neurosci. 21, 9844–9855 (2001).
Linke, R., De Lima, A. D., Schwegler, H. & Pape, H. C. Direct synaptic connections of axons from superior colliculus with identified thalamo-amygdaloid projection neurons in the rat: possible substrates of a subcortical visual pathway to the amygdala. J. Comp. Neurol. 403, 158–170 (1999).
Fanselow, M. S. Contextual fear, Gestalt memories, and the hippocampus. Behav. Brain Res. 110, 73–81 (2000).
Anagnostaras, S. G., Gale, G. D. & Fanselow, M. S. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus 11, 8–17 (2001).
Mascagni, F., McDonald, A. J. & Coleman, J. R. Corticoamygdaloid and corticocortical projections of the rat temporal cortex: a Phaseolus vulgaris leucoagglutinin study. Neuroscience 57, 697–715 (1993).
Romanski, L. M. & LeDoux, J. E. Information cascade from primary auditory cortex to the amygdala: corticocortical and corticoamygdaloid projections of temporal cortex in the rat. Cereb. Cortex 3, 515–532 (1993).
LeDoux, J. E., Farb, C. & Ruggiero, D. A. Topographic organization of neurons in the acoustic thalamus that project to the amygdala. J. Neurosci. 10, 1043–1054 (1990).
Amaral, D. G., Price, J. L., Pitkänen, A. & Carmichael, S. T. in The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction (ed. Aggleton, J. P.) 1–66 (Wiley–Liss, New York, 1992).
McDonald, A. J. Cortical pathways to the mammalian amygdala. Prog. Neurobiol. 55, 257–332 (1998).
LeDoux, J. E., Cicchetti, P., Xagoraris, A. & Romanski, L. M. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J. Neurosci. 10, 1062–1069 (1990).The first demonstration of the role of the LA in fear conditioning. It shifted the emphasis from the central to the lateral nucleus in the search for sites of plasticity.
Goosens, K. A. & Maren, S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn. Mem. 8, 148–155 (2001).
Doron, N. N. & LeDoux, J. E. Cells in the posterior thalamus project to both amygdala and temporal cortex: a quantitative retrograde double-labeling study in the rat. J. Comp. Neurol. 425, 257–274 (2000).
Romanski, L. M. & LeDoux, J. E. Equipotentiality of thalamo-amygdala and thalamo-cortico-amygdala projections as auditory conditioned stimulus pathways. J. Neurosci. 12, 4501–4509 (1992).
Jarrell, T. W., Gentile, C. G., Romanski, L. M., McCabe, P. M. & Schneiderman, N. Involvement of cortical and thalamic auditory regions in retention of differential bradycardia conditioning to acoustic conditioned stimuli in rabbits. Brain Res. 412, 285–294 (1987).
Armony, J. L., Servan-Schreiber, D., Romanski, L. M., Cohen, J. D. & LeDoux, J. E. Stimulus generalization of fear responses: effects of auditory cortex lesions in a computational model and in rats. Cereb. Cortex 7, 157–165 (1997).
Campeau, S. & Davis, M. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J. Neurosci. 15, 2301–2311 (1995).
Shi, C. & Davis, M. Pain pathways involved in fear conditioning measured with fear-potentiated startle: lesion studies. J. Neurosci. 19, 420–430 (1999).The first attempt to map the US pathways to the amygdala. Indicates that as in the case of the auditory CS pathways, the US reaches the amygdala by way of thalamic and cortical somatosensory routes.
Quirk, G. J., Repa, J. C. & LeDoux, J. E. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron 15, 1029–1039 (1995).The first demonstration of cellular plasticity in the LA. Latencies indicated a role for direct thalamic inputs to the LA from the auditory thalamus.
Quirk, G. J., Armony, J. L. & LeDoux, J. E. Fear conditioning enhances different temporal components of toned-evoked spike trains in auditory cortex and lateral amygdala. Neuron 19, 613–624 (1997).
Morris, J. S., Ohman, A. & Dolan, R. J. A subcortical pathway to the right amygdala mediating 'unseen' fear. Proc. Natl Acad. Sci. USA 96, 1680–1685 (1999).Presented evidence for the existence of a subcortical CS transmission route to the amygdala in the human brain, mirroring the findings in rats.
LeDoux, J. E., Ruggiero, D. A., Forest, R., Stornetta, R. & Reis, D. J. Topographic organization of convergent projections to the thalamus from the inferior colliculus and spinal cord in the rat. J. Comp. Neurol. 264, 123–146 (1987).
Romanski, L. M., LeDoux, J. E., Clugnet, M. C. & Bordi, F. Somatosensory and auditory convergence in the lateral nucleus of the amygdala. Behav. Neurosci. 107, 444–450 (1993).
Turner, B. H. & Zimmer, J. The architecture and some interconnections of the rat amygdala and lateral periallocortex. J. Comp. Neurol. 227, 540–557 (1984).
Bernard, J. F. & Besson, J. M. The spino(trigemino)pontoamygdaloid pathway: electrophysiological evidence for an involvement in pain processes. J. Neurophysiol. 63, 473–489 (1990).
Burstein, R. & Potrebic, S. Retrograde labeling of neurons in the spinal cord that project directly to the amygdala or the orbital cortex in the rat. J. Comp. Neurol. 335, 469–485 (1993).
Pitkanen, A., Savander, V. & LeDoux, J. E. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 20, 517–523 (1997).
Kapp, B. S., Whalen, P. J., Supple, W. F. & Pascoe, J. P. in The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction (ed. Aggleton, J. P.) 229–254 (Wiley–Liss, New York, 1992).
LeDoux, J. E., Iwata, J., Cicchetti, P. & Reis, D. J. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci. 8, 2517–2529 (1988).
Gentile, C. G., Jarrell, T. W., Teich, A., McCabe, P. M. & Schneiderman, N. The role of amygdaloid central nucleus in the retention of differential Pavlovian conditioning of bradycardia in rabbits. Behav. Brain Res. 20, 263–273 (1986).
Davis, M. The role of the amygdala in fear-potentiated startle: implications for animal models of anxiety. Trends Pharmacol. Sci. 13, 35–41 (1992).
Hitchcock, J. & Davis, M. Lesions of the amygdala but not of the cerebellum or red nucleus block conditioned fear as measured with the potentiated startle paradigm. Behav. Neurosci. 100, 11–22 (1986).
Iwata, J., LeDoux, J. E., Meeley, M. P., Arneric, S. & Reis, D. J. Intrinsic neurons in the amygdaloid field projected to by the medial geniculate body mediate emotional responses conditioned to acoustic stimuli. Brain Res. 383, 195–214 (1986).
Kapp, B. S., Frysinger, R. C., Gallagher, M. & Haselton, J. Amygdala central nucleus lesions: effect on heart rate conditioning in the rabbit. Physiol. Behav. 23, 1109–1117 (1979).The first clear demonstration of the role of the amygdala in auditory fear conditioning, and the first clear indication that an amygdala region was involved in such learning by virtue of its anatomical connectivity with areas that control CRs — autonomic responses in this case.
Van de Kar, L. D., Piechowski, R. A., Rittenhouse, P. A. & Gray, T. S. Amygdaloid lesions: differential effect on conditioned stress and immobilization-induced increases in corticosterone and renin secretion. Neuroendocrinology 54, 89–95 (1991).
Pare, D. & Smith, Y. Intrinsic circuitry of the amygdaloid complex: common principles of organization in rats and cats. Trends Neurosci. 21, 240–241 (1998).
Collins, D. R. & Pare, D. Reciprocal changes in the firing probability of lateral and central medial amygdala neurons. J. Neurosci. 19, 836–844 (1999).
Royer, S., Martina, M. & Pare, D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J. Neurosci. 19, 10575–10583 (1999).
Nader, K., Majidishad, P., Amorapanth, P. & LeDoux, J. E. Damage to the lateral and central, but not other, amygdaloid nuclei prevents the acquisition of auditory fear conditioning. Learn. Mem. 8, 156–163 (2001).
Amorapanth, P., LeDoux, J. E. & Nader, K. Different lateral amygdala outputs mediate reactions and actions elicited by a fear-arousing stimulus. Nature Neurosci. 3, 74–79 (2000).
Majidishad, P., Pelli, D. G. & LeDoux, J. E. Disruption of fear conditioning to contextual stimuli but not to a tone by lesions of the accessory basal nucleus of the amygdala. Soc. Neurosci. Abstr. 22, 1116 (1996).
Killcross, S., Robbins, T. W. & Everitt, B. J. Different types of fear-conditioned behavior mediated by separate nuclei within amygdala. Nature 388, 377–380 (1997).Showed that different routes of information flow in the amygdala might be involved in the control of different types of fear-related response, especially reflexive responses.
Nader, K. & LeDoux, J. E. Is it time in invoke multiple fear learning systems? Trends Cogn. Sci. 1, 241–244 (1997).
Marquis, D. G. & Hilgard, E. R. Conditioned lid responses to light in dogs after removal of the visual cortex. J. Comp. Psychol. 22, 157–178 (1936).
Mauk, M. D. & Thompson, R. F. Retention of classically conditioned eyelid responses following acute decerebration. Brain Res. 403, 89–95 (1987).
Norman, R. J., Buchwald, J. S. & Villablanca, J. R. Classical conditioning with auditory discrimination of the eye blink in decerebrate cats. Science 196, 551–553 (1977).
McCormick, D. A., Clark, G. A., Lavond, D. G. & Thompson, R. F. Initial localization of the memory trace for a basic form of learning. Proc. Natl Acad. Sci. USA 79, 2731–2735 (1982).
Welsh, J. P. & Harvey, J. A. Cerebellar lesions and the nictitating membrane reflex: performance deficits of the conditioned and unconditioned response. J. Neurosci. 9, 299–311 (1989).
Bloedel, J. R. & Bracha, V. On the cerebellum, cutaneomuscular reflexes, movement control and the elusive engrams of memory. Behav. Brain Res. 68, 1–44 (1995).
Steinmetz, J. E. et al. Disruption of classical eyelid conditioning after cerebellar lesions: damage to a memory trace system or a simple performance deficit? J. Neurosci. 12, 4403–4426 (1992).
Krupa, D. J., Thompson, J. K. & Thompson, R. F. Localization of a memory trace in the mammalian brain. Science 260, 989–991 (1993).
Nordholm, A. F., Thompson, J. K., Dersarkissian, C. & Thompson, R. F. Lidocaine infusion in a critical region of cerebellum completely prevents learning of the conditioned eyeblink response. Behav. Neurosci. 107, 882–886 (1993).
Attwell, P. J., Rahman, S. & Yeo, C. H. Acquisition of eyeblink conditioning is critically dependent on normal function in cerebellar cortical lobule HVI. J. Neurosci. 21, 5715–5722 (2001).Pharmacological inactivation of the cerebellar cortex prevented both the acquisition of new responses and the expression of previously acquired responses. By using a reversible lesion technique, the authors were able to rule out a simple performance deficit as the cause of the observed impairment in learning.
Krupa, D. J., Weng, J. & Thompson, R. F. Inactivation of brainstem motor nuclei blocks expression but not acquisition of the rabbit's classically conditioned eyeblink response. Behav. Neurosci. 110, 219–227 (1996).
Krupa, D. J. & Thompson, R. F. Inactivation of the superior cerebellar peduncle blocks expression but not acquisition of the rabbit's classically conditioned eye-blink response. Proc. Natl Acad. Sci. USA 92, 5097–5101 (1995).
Yeo, C. H., Hardiman, M. J. & Glickstein, M. Classical conditioning of the nictitating membrane response of the rabbit. II. Lesions of the cerebellar cortex. Exp. Brain Res. 60, 99–113 (1985).
Yeo, C. H., Hardiman, M. J. & Glickstein, M. Discrete lesions of the cerebellar cortex abolish the classically conditioned nictitating membrane response of the rabbit. Behav. Brain Res. 13, 261–266 (1984).
Perrett, S. P., Ruiz, B. P. & Mauk, M. D. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. J. Neurosci. 13, 1708–1718 (1993).
Garcia, K. S., Steele, P. M. & Mauk, M. D. Cerebellar cortex lesions prevent acquisition of conditioned eyelid responses. J. Neurosci. 19, 10940–10947 (1999).When cerebellar cortex lesions caused a disruption of response to a previously trained CS1, the same lesions prevented acquisition of responses to a different CS2.
Blanchard, C. D. & Blanchard, R. J. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J. Comp. Physiol. Psychol. 81, 281–290 (1972).
LeDoux, J. E. in The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction (ed. Aggleton, J. P.) 339–351 (Wiley–Liss, New York, 1992).
LeDoux, J. E., Farb, C. & Romanski, L. Overlapping projections to the amygdala and striatum from auditory processing areas of the thalamus and cortex. Neurosci. Lett. 134, 139–144 (1991).
Bordi, F. & LeDoux, J. Sensory tuning beyond the sensory system: an initial analysis of auditory properties of neurons in the lateral amygdaloid nucleus and overlying areas of the striatum. J. Neurosci. 12, 2493–2503 (1992).
Clugnet, M. C., LeDoux, J. E. & Morrison, S. F. Unit responses evoked in the amygdala and striatum by electrical stimulation of the medial geniculate body. J. Neurosci. 10, 1055–1061 (1990).
Helmstetter, F. J. & Bellgowan, P. S. Effects of muscimol applied to the basolateral amygdala on acquisition and expression of contextual fear conditioning in rats. Behav. Neurosci. 108, 1005–1009 (1994).
Muller, J., Corodimas, K. P., Fridel, Z. & LeDoux, J. E. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav. Neurosci. 111, 683–691 (1997).
McGaugh, J. L. Memory — a century of consolidation. Science 287, 248–251 (2000).
Cahill, L., Weinberger, N. M., Roozendaal, B. & McGaugh, J. L. Is the amygdala a locus of 'conditioned fear'? Some questions and caveats. Neuron 23, 227–228 (1999).
Wilensky, A. E., Schafe, G. E. & LeDoux, J. E. Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. J. Neurosci. 19, RC48 (1999).
Wilensky, A. E., Schafe, G. E. & LeDoux, J. E. The amygdala modulates memory consolidation of fear-motivated inhibitory avoidance learning but not classical fear conditioning. J. Neurosci. 20, 7059–7066 (2000).
Wallace, K. J. & Rosen, J. B. Neurotoxic lesions of the lateral nucleus of the amygdala decrease conditioned fear but not unconditioned fear of a predator odor: comparison with electrolytic lesions. J. Neurosci. 21, 3619–3627 (2001).
Gould, T. J. & Steinmetz, J. E. Changes in rabbit cerebellar cortical and interpositus nucleus activity during acquisition, extinction, and backward classical eyelid conditioning. Neurobiol. Learn. Mem. 65, 17–34 (1996).
Logan, C. G. & Grafton, S. T. Functional anatomy of human eyeblink conditioning determined with regional cerebral glucose metabolism and positron-emission tomography. Proc. Natl Acad. Sci. USA 92, 7500–7504 (1995).
Ramnani, N., Toni, I., Josephs, O., Ashburner, J. & Passingham, R. E. Learning- and expectation-related changes in the human brain during motor learning. J. Neurophysiol. 84, 3026–3035 (2000).
Schreurs, B. G. et al. Lateralization and behavioral correlation of changes in regional cerebral blood flow with classical conditioning of the human eyeblink response. J. Neurophysiol. 77, 2153–2163 (1997).
Hansel, C., Linden, D. J. & D'Angelo, E. Beyond parallel fiber LTD: the diversity of synaptic and non-synaptic plasticity in the cerebellum. Nature Neurosci. 4, 467–475 (2001).
Aizenman, C. D. & Linden, D. J. Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nature Neurosci. 3, 109–111 (2000).
Racine, R. J., Wilson, D. A., Gingell, R. & Sunderland, D. Long-term potentiation in the interpositus and vestibular nuclei in the rat. Exp. Brain Res. 63, 158–162 (1986).
Repa, J. C. et al. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nature Neurosci. 4, 724–731 (2001).Discovered different populations of cells that are involved in triggering and storing physiological changes in LA subregions during fear conditioning.
Rogan, M., Staubli, U. & LeDoux, J. Fear conditioning induces associative long-term potentiation in the amygdala. Nature 390, 604–607 (1997).
Pare, D. & Collins, D. R. Neuronal correlates of fear in the lateral amygdala: multiple extracellular recordings in conscious cats. J. Neurosci. 20, 2701–2710 (2000).
Maren, S. Auditory fear conditioning increases CS-elicited spike firing in lateral amygdala neurons even after extensive overtraining. Eur. J. Neurosci. 12, 4047–4054 (2000).
McKernan, M. G. & Shinnick-Gallagher, P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature 390, 607–611 (1997).
Rogan, M. T., Weisskopf, M. G., Huang, Y.-Y., Kandel, E. R. & LeDoux, J. E. in Neuronal Mechanisms of Memory Formation (ed. Hölscher, C.) 58–76 (Cambridge Univ. Press, Cambridge, 2001).
Schafe, G. E., Nader, K., Blair, H. T. & LeDoux, J. E. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci. 24, 540–546 (2001).
LaBar, K. S., Gatenby, J. C., Gore, J. C., LeDoux, J. E. & Phelps, E. A. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron 20, 937–945 (1998).
Buchel, C., Morris, J., Dolan, R. J. & Friston, K. J. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron 20, 947–957 (1998).
Morris, J. S., Buchel, C. & Dolan, R. J. Parallel neural responses in amygdala subregions and sensory cortex during implicit fear conditioning. Neuroimage 13, 1044–1052 (2001).
Phelps, E. A. et al. Activation of the left amygdala to a cognitive representation of fear. Nature Neurosci. 4, 437–441 (2001).
Ohyama, T. & Mauk, M. Latent acquisition of timed responses in cerebellar cortex. J. Neurosci. 21, 682–690 (2001).Presented evidence indicating that during eyelid conditioning, plasticity in the cerebellar cortex is induced before any behavioral responses are evident.
Schafe, G. E. et al. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of Pavlovian fear conditioning. J. Neurosci. 20, 8177–8187 (2000).
Weinberger, N. M. Physiological memory in primary auditory cortex: characteristics and mechanisms. Neurobiol. Learn. Mem. 70, 226–251 (1998).
Armony, J. L., Quirk, G. J. & LeDoux, J. E. Differential effects of amygdala lesions on early and late plastic components of auditory cortex spiketrains during fear conditioning. J. Neurosci. 18, 2592–2601 (1998).
Poremba, A. & Gabriel, M. Amygdalar efferents initiate auditory thalamic discriminative training-induced neuronal activity. J. Neurosci. 21, 270–278 (2001).
Maren, S., Yap, S. A. & Goosens, K. A. The amygdala is essential for the development of neuronal plasticity in the medial geniculate nucleus during auditory fear conditioning in rats. J. Neurosci. 21, RC135 (2001).
Garcia, K. S. & Mauk, M. D. Pharmacological analysis of cerebellar contributions to the timing and expression of conditioned eyelid responses. Neuropharmacology 37, 471–480 (1998).
Chen, L., Bao, S. & Thompson, R. F. Bilateral lesions of the interpositus nucleus completely prevent eyeblink conditioning in Purkinje cell-degeneration mutant mice. Behav. Neurosci. 113, 204–210 (1999).
Chen, L., Bao, S., Lockard, J. M., Kim, J. K. & Thompson, R. F. Impaired classical eyeblink conditioning in cerebellar-lesioned and Purkinje cell degeneration (pcd) mutant mice. J. Neurosci. 16, 2829–2838 (1996).This study provides some of the strongest evidence in support of the idea that learning of the conditioned eyelid response does not require the cerebellar cortex.
Miyata, M. et al. Deficient long-term synaptic depression in the rostral cerebellum correlated with impaired motor learning in phospholipase C β4 mutant mice. Eur. J. Neurosci. 13, 1945–1954 (2001).In contrast to the pcd mice (Reference 140 ), mice with deficient cerebellar LTD could not learn the conditioned eyelid response, even though other properties of the granule-to-Purkinje synapse seemed to be normal.
Perrett, S. P. & Mauk, M. D. Extinction of conditioned eyelid responses requires the anterior lobe of cerebellar cortex. J. Neurosci. 15, 2074–2080 (1995).
Medina, J. F., Nores, W. L., Ohyama, T. & Mauk, M. D. Mechanisms of cerebellar learning suggested by eyelid conditioning. Curr. Opin. Neurobiol. 10, 717–724 (2000).
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- EXTINCTION
-
The reduction and cessation of a predictive relationship and behaviour after the omission of a reinforcer (negative prediction error).
- GOLGI CELLS
-
Cerebellar interneurons located in the granule cell layer. Their axonal terminals form part of the cerebellar glomeruli.
- PURKINJE CELLS
-
Inhibitory interneurons in the cerebellum that use GABA as their neurotransmitter. Their cell bodies are situated beneath the molecular layer, and their dendrites branch extensively in this layer. Their axons project into the underlying white matter, and they provide the only output from the cerebellar cortex.
- INFERIOR OLIVARY NUCLEUS
-
A nucleus situated in a bulge on the ventral medullary surface of the brainstem. Its neurons form very strong excitatory synapses with those of the cerebellum.
- PARABRACHIAL AREA
-
A nucleus situated in the pons that transmits information from the viscera to the hypothalamus and amygdala.
- STRIA TERMINALIS
-
One of the main efferent projections of the amygdala. It innervates regions that include the nucleus accumbens and the hypothalamus.
- MITOGEN-ACTIVATED PROTEIN KINASE
-
Any member of a family of protein kinases that are important for relaying signals from the cell membrane to the nucleus.
- PROTEIN KINASE A
-
Also known as cyclic-AMP-dependent protein kinase. One of a class of enzymes that use ATP as a phosphoryl-group donor to phosphorylate hydroxyl or phenolic groups on their target proteins.
Rights and permissions
About this article
Cite this article
Medina, J., Christopher Repa, J., Mauk, M. et al. Parallels between cerebellum- and amygdala-dependent conditioning. Nat Rev Neurosci 3, 122–131 (2002). https://doi.org/10.1038/nrn728
Issue Date:
DOI: https://doi.org/10.1038/nrn728
This article is cited by
-
Local synaptic inhibition mediates cerebellar granule cell pattern separation and enables learned sensorimotor associations
Nature Neuroscience (2024)
-
Pyk2 in the amygdala modulates chronic stress sequelae via PSD-95-related micro-structural changes
Translational Psychiatry (2019)
-
Sound check, stage design and screen plot – how to increase the comparability of fear conditioning and fear extinction experiments
Psychopharmacology (2019)
-
Extinction and Renewal of Conditioned Eyeblink Responses in Focal Cerebellar Disease
The Cerebellum (2019)
-
Mutation of the HERC 1 Ubiquitin Ligase Impairs Associative Learning in the Lateral Amygdala
Molecular Neurobiology (2018)