Abstract
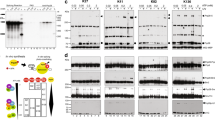
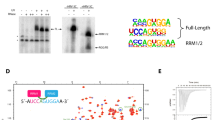
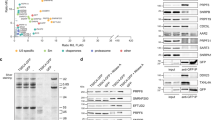
Several protein kinases, including SRPK1 and SRPK2, have been implicated in spliceosome assembly and catalytic activation. However, little is known about their targets. Here we show that SRPK1 is predominantly associated with U1 small nuclear ribonucleoprotein (snRNP), whereas SRPK2 associates with the U4/U6-U5 tri-snRNP. RNAi-mediated depletion in HeLa cells showed that SRPK2 is essential for cell viability, and it is required for spliceosomal B complex formation. SRPK2 knock down results in hypophosphorylation of the arginine-serine (RS) domain–containing human PRP28 protein (PRP28, also known as DDX23), and destabilizes PRP28 association with the tri-snRNP. Immunodepletion of PRP28 from HeLa cell nuclear extract and complementation studies revealed that PRP28 phosphorylation is required for its stable association with the tri-snRNP and for tri-snRNP integration into the B complex. Our results demonstrate a role for SRPK2 in splicing and reveal a previously unknown function for PRP28 in spliceosome assembly.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Jurica, M.S. & Moore, M.J. Pre-mRNA splicing: awash in a sea of proteins. Mol. Cell 12, 5–14 (2003).
Will, C.L. & Lührmann, R. Spliceosome structure and function. in The RNA World 3rd edn (eds. Gesteland, R.F., Cech, T.R. & Atkins, J.F.) 369–400 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 2006).
Staley, J.P. & Guthrie, C. Mechanical devices of the spliceosome: motors, clocks, springs, and things. Cell 92, 315–326 (1998).
Fu, X.D. The superfamily of arginine/serine-rich splicing factors. RNA 1, 663–680 (1995).
Graveley, B.R. Sorting out the complexity of SR protein functions. RNA 6, 1197–1211 (2000).
Sanford, J.R., Ellis, J. & Cáceres, J.F. Multiple roles of arginine/serine-rich splicing factors in RNA processing. Biochem. Soc. Trans. 33, 443–446 (2005).
Black, D.L. Mechanisms of alternative pre-messenger RNA splicing. Annu. Rev. Biochem. 72, 291–336 (2003).
Wu, J.Y. & Maniatis, T. Specific interactions between proteins implicated in splice site selection and regulated alternative splicing. Cell 75, 1061–1070 (1993).
Shen, H. & Green, M.R. A pathway of sequential arginine-serine-rich domain-splicing signal interactions during mammalian spliceosome assembly. Mol. Cell 16, 363–373 (2004).
Shen, H. & Green, M.R. RS domain-splicing signal interactions in splicing of U12-type and U2-type introns. Nat. Struct. Mol. Biol. 14, 597–603 (2007).
Teigelkamp, S., Mundt, C., Achsel, T., Will, C.L. & Lührmann, R. The human U5 snRNP-specific 100-kD protein is an RS domain-containing, putative RNA helicase with significant homology to the yeast splicing factor Prp28p. RNA 3, 1313–1326 (1997).
Woppmann, A. et al. Identification of an snRNP-associated kinase activity that phosphorylates arginine/serine rich domains typical of splicing factors. Nucleic Acids Res. 21, 2815–2822 (1993).
Staley, J.P. & Guthrie, C. An RNA switch at the 5′ splice site requires ATP and the DEAD box protein Prp28p. Mol. Cell 3, 55–64 (1999).
Will, C.L. et al. Characterization of novel SF3b and 17S U2 snRNP proteins, including a human Prp5p homologue and an SF3b DEAD-box protein. EMBO J. 21, 4978–4988 (2002).
Jamison, S.F. et al. U1 snRNP-ASF/SF2 interaction and 5′ splice site recognition: characterization of required elements. Nucleic Acids Res. 23, 3260–3267 (1995).
Kohtz, J.D. et al. Protein-protein interactions and 5′-splice-site recognition in mammalian mRNA precursors. Nature 368, 119–124 (1994).
Eperon, I.C. et al. Selection of alternative 5′ splice sites: role of U1 snRNP and models for the antagonistic effects of SF2/ASF and hnRNP A1. Mol. Cell. Biol. 20, 8303–8318 (2000).
Roscigno, R.F. & Garcia-Blanco, M.A. SR proteins escort the U4/U6·U5 tri-snRNP to the spliceosome. RNA 1, 692–706 (1995).
Makarova, O.V., Makarov, E.M. & Lührmann, R. The 65 and 110 kDa SR-related proteins of the U4/U6·U5 tri-snRNP are essential for the assembly of mature spliceosomes. EMBO J. 20, 2553–2563 (2001).
Mermoud, J.E., Cohen, P. & Lamond, A.I. Ser/Thr-specific protein phosphatases are required for both catalytic steps of pre-mRNA splicing. Nucleic Acids Res. 20, 5263–5269 (1992).
Mermoud, J.E., Cohen, P.T. & Lamond, A.I. Regulation of mammalian spliceosome assembly by a protein phosphorylation mechanism. EMBO J. 13, 5679–5688 (1994).
Manley, J.L. & Tacke, R. SR proteins and splicing control. Genes Dev. 10, 1569–1579 (1996).
Xiao, S.H. & Manley, J.L. Phosphorylation of the ASF/SF2 RS domain affects both protein-protein and protein-RNA interactions and is necessary for splicing. Genes Dev. 11, 334–344 (1997).
Shi, Y., Reddy, B. & Manley, J.L. PP1/PP2A phosphatases are required for the second step of Pre-mRNA splicing and target specific snRNP proteins. Mol. Cell 23, 819–829 (2006).
Colwill, K. et al. The Clk/Sty protein kinase phosphorylates SR splicing factors and regulates their intranuclear distribution. EMBO J. 15, 265–275 (1996).
Rossi, F. et al. Specific phosphorylation of SR proteins by mammalian DNA topoisomerase I. Nature 381, 80–82 (1996).
Gui, J.F., Lane, W.S. & Fu, X.D. A serine kinase regulates intracellular localization of splicing factors in the cell cycle. Nature 369, 678–682 (1994).
Kuroyanagi, N., Onogi, H., Wakabayashi, T. & Hagiwara, M. Novel SR-protein-specific kinase, SRPK2, disassembles nuclear speckles. Biochem. Biophys. Res. Commun. 242, 357–364 (1998).
Wang, H.Y. et al. SRPK2: a differentially expressed SR protein-specific kinase involved in mediating the interaction and localization of pre-mRNA splicing factors in mammalian cells. J. Cell Biol. 140, 737–750 (1998).
Nakagawa, O. et al. Centronuclear myopathy in mice lacking a novel muscle-specific protein kinase transcriptionally regulated by MEF2. Genes Dev. 19, 2066–2077 (2005).
Fetzer, S., Lauber, J., Will, C.L. & Lührmann, R. The [U4/U6·U5] tri-snRNP-specific 27K protein is a novel SR protein that can be phosphorylated by the snRNP-associated protein kinase. RNA 3, 344–355 (1997).
Lemm, I. et al. Ongoing U snRNP biogenesis is required for the integrity of Cajal bodies. Mol. Biol. Cell 17, 3221–3231 (2006).
Lee, K.A. & Green, M.R. Small-scale preparation of extracts from radiolabeled cells efficient in pre-mRNA splicing. Methods Enzymol. 181, 20–30 (1990).
Makarova, O.V., Makarov, E.M., Liu, S., Vornlocher, H.-P. & Lührmann, R. Protein 61K, encoded by a gene (PRPF31) linked to autosomal dominant retinitis pigmentosa, is required for U4/U6·U5 tri-snRNP formation and pre-mRNA splicing. EMBO J. 21, 1148–1157 (2002).
Schaffert, N., Hossbach, M., Heintzmann, R., Achsel, T. & Lührmann, R. RNAi knockdown of hPrp31 leads to an accumulation of U4/U6 di-snRNPs in Cajal bodies. EMBO J. 23, 3000–3009 (2004).
Laggerbauer, B. et al. The human U5 snRNP 52K protein (CD2BP2) interacts with U5-102K (hPrp6), a U4/U6·U5 tri-snRNP bridging protein, but dissociates upon tri-snRNP formation. RNA 11, 598–608 (2005).
Daub, H. et al. Identification of SRPK1 and SRPK2 as the major cellular protein kinases phosphorylating hepatitis B virus core protein. J. Virol. 76, 8124–8137 (2002).
Kamachi, M. et al. Human autoimmune sera as molecular probes for the identification of an autoantigen kinase signaling pathway. J. Exp. Med. 196, 1213–1225 (2002).
Woppmann, A., Patschinsky, T., Bringmann, P., Godt, F. & Lührmann, R. Characterisation of human and murine snRNP proteins by two-dimensional gel electrophoresis and phosphopeptide analysis of U1-specific 70K protein variants. Nucleic Acids Res. 18, 4427–4438 (1990).
Stoyanov, A.V. & Righetti, P.G. Dynamics of protein isoelectric focusing in immobilized pH gradient gels. Electrophoresis 17, 1313–1318 (1996).
Liu, S., Rauhut, R., Vornlocher, H.P. & Lührmann, R. The network of protein-protein interactions within the human U4/U6·U5 tri-snRNP. RNA 12, 1418–1430 (2006).
Strauss, E.J. & Guthrie, C. A cold-sensitive mRNA splicing mutant is a member of the RNA helicase gene family. Genes Dev. 5, 629–641 (1991).
Strauss, E.J. & Guthrie, C. PRP28, a 'DEAD-box' protein, is required for the first step of mRNA splicing in vitro. Nucleic Acids Res. 22, 3187–3193 (1994).
Tazi, J. et al. Thiophosphorylation of U1–70K protein inhibits pre-mRNA splicing. Nature 363, 283–286 (1993).
Bessonov, S. et al. Isolation of an active step I spliceosome and composition of its RNP core. Nature advance online publication, doi:10.1038/nature06842 (5 March 2008).
Elbashir, S.M., Harborth, J., Weber, K. & Tuschl, T. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods 26, 199–213 (2002).
Lerner, E.A., Lerner, M.R., Janeway, C.A. Jr & Steitz, J.A. Monoclonal antibodies to nucleic acid-containing cellular constituents: probes for molecular biology and autoimmune disease. Proc. Natl. Acad. Sci. USA 78, 2737–2741 (1981).
Kastner, B. & Lührmann, R. Purification of U small nuclear ribonucleoprotein particles. Methods Mol. Biol. 118, 289–298 (1999).
Shin, C. & Manley, J.L. The SR protein SRp38 represses splicing in M phase cells. Cell 111, 407–417 (2002).
Dignani, J.D., Lebovitz, R.M. & Roeder, R.G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11, 1475–1489 (1983).
Nikolakaki, E. et al. Cloning and characterization of an alternatively spliced form of SR protein kinase 1 that interacts specifically with scaffold attachment factor-B. J. Biol. Chem. 276, 40175–40182 (2001).
Acknowledgements
We thank I. Lemm and M. Hoßbach for help with RNAi experiments, X.-D. Fu (University of California, San Diego, USA) for the plasmid encoding SRPK2, H. Manniga for synthesis of siRNAs, and S. Trowitzsch and G. Weber for the gift of purified tri-snRNP. We thank C.L. Will for critically reading the manuscript. We acknowledge the expert technical assistance of H. Kohansal, P. Kempkes and T. Conrad with HeLa cell extract and snRNP preparation, and M. Raabe with MS. This work was funded by grants from the DFG, the Fonds der Chemischen Industrie, the Volkswagen Stiftung and the EURASNET (within the 6th EU framework; LSHG-CT-2005-518238).
Author information
Authors and Affiliations
Contributions
R.M., K.H. and R.L. designed the research; R.M. performed the research; S.M. and R.F. provided recombinant PRP28; H.U. performed MS; R.M., K.H. and R.L. analyzed data and wrote the paper.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Materials (PDF 1850 kb)
Rights and permissions
About this article
Cite this article
Mathew, R., Hartmuth, K., Möhlmann, S. et al. Phosphorylation of human PRP28 by SRPK2 is required for integration of the U4/U6-U5 tri-snRNP into the spliceosome. Nat Struct Mol Biol 15, 435–443 (2008). https://doi.org/10.1038/nsmb.1415
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.1415
This article is cited by
-
Novel thrombospondin-1 transcript exhibits distinctive expression and activity in thyroid tumorigenesis
Oncogene (2023)
-
Activation of Prp28 ATPase by phosphorylated Npl3 at a critical step of spliceosome remodeling
Nature Communications (2021)
-
A bioinformatic analysis identifies circadian expression of splicing factors and time-dependent alternative splicing events in the HD-MY-Z cell line
Scientific Reports (2019)
-
Comparison of village dog and wolf genomes highlights the role of the neural crest in dog domestication
BMC Biology (2018)
-
Structures of the human pre-catalytic spliceosome and its precursor spliceosome
Cell Research (2018)