Abstract
Rapid neurotransmitter release depends on the Ca2+ sensor Synaptotagmin-1 (Syt1) and the SNARE complex formed by synaptobrevin, syntaxin-1 and SNAP-25. How Syt1 triggers release has been unclear, partly because elucidating high-resolution structures of Syt1–SNARE complexes has been challenging. An NMR approach based on lanthanide-induced pseudocontact shifts now reveals a dynamic binding mode in which basic residues in the concave side of the Syt1 C2B-domain β-sandwich interact with a polyacidic region of the SNARE complex formed by syntaxin-1 and SNAP-25. The physiological relevance of this dynamic structural model is supported by mutations in basic residues of Syt1 that markedly impair SNARE-complex binding in vitro and Syt1 function in neurons. Mutations with milder effects on binding have correspondingly milder effects on Syt1 function. Our results support a model whereby dynamic interaction facilitates cooperation between Syt1 and the SNAREs in inducing membrane fusion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Brunger, A.T., Weninger, K., Bowen, M. & Chu, S. Single-molecule studies of the neuronal SNARE fusion machinery. Annu. Rev. Biochem. 78, 903–928 (2009).
Rizo, J. & Sudhof, T.C. The membrane fusion enigma: SNAREs, Sec1/Munc18 proteins, and their accomplices—guilty as charged? Annu. Rev. Cell Dev. Biol. 28, 279–308 (2012).
Poirier, M.A. et al. The synaptic SNARE complex is a parallel four-stranded helical bundle. Nat. Struct. Biol. 5, 765–769 (1998).
Sutton, R.B., Fasshauer, D., Jahn, R. & Brunger, A.T. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395, 347–353 (1998).
Hanson, P.I., Roth, R., Morisaki, H., Jahn, R. & Heuser, J.E. Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell 90, 523–535 (1997).
Fernández-Chacón, R. et al. Synaptotagmin I functions as a calcium regulator of release probability. Nature 410, 41–49 (2001).
Sutton, R.B., Davletov, B.A., Berghuis, A.M., Sudhof, T.C. & Sprang, S.R. Structure of the first C2 domain of synaptotagmin I: a novel Ca2+/phospholipid-binding fold. Cell 80, 929–938 (1995).
Ubach, J., Zhang, X., Shao, X., Sudhof, T.C. & Rizo, J. Ca2+ binding to synaptotagmin: how many Ca2+ ions bind to the tip of a C2-domain? EMBO J. 17, 3921–3930 (1998).
Fernandez, I. et al. Three-dimensional structure of the synaptotagmin 1 C2B-domain: synaptotagmin 1 as a phospholipid binding machine. Neuron 32, 1057–1069 (2001).
Mackler, J.M. & Reist, N.E. Mutations in the second C2 domain of synaptotagmin disrupt synaptic transmission at Drosophila neuromuscular junctions. J. Comp. Neurol. 436, 4–16 (2001).
Araç, D. et al. Close membrane-membrane proximity induced by Ca2+-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat. Struct. Mol. Biol. 13, 209–217 (2006).
Xue, M., Ma, C., Craig, T.K., Rosenmund, C. & Rizo, J. The Janus-faced nature of the C2B domain is fundamental for synaptotagmin-1 function. Nat. Struct. Mol. Biol. 15, 1160–1168 (2008).
Pang, Z.P., Shin, O.H., Meyer, A.C., Rosenmund, C. & Sudhof, T.C. A gain-of-function mutation in synaptotagmin-1 reveals a critical role of Ca2+-dependent soluble N-ethylmaleimide-sensitive factor attachment protein receptor complex binding in synaptic exocytosis. J. Neurosci. 26, 12556–12565 (2006).
Tang, J. et al. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell 126, 1175–1187 (2006).
Schaub, J.R., Lu, X., Doneske, B., Shin, Y.K. & McNew, J.A. Hemifusion arrest by complexin is relieved by Ca2+–synaptotagmin I. Nat. Struct. Mol. Biol. 13, 748–750 (2006).
Giraudo, C.G., Eng, W.S., Melia, T.J. & Rothman, J.E. A clamping mechanism involved in SNARE-dependent exocytosis. Science 313, 676–680 (2006).
Reim, K. et al. Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell 104, 71–81 (2001).
Huntwork, S. & Littleton, J.T. A complexin fusion clamp regulates spontaneous neurotransmitter release and synaptic growth. Nat. Neurosci. 10, 1235–1237 (2007).
Maximov, A., Tang, J., Yang, X., Pang, Z.P. & Sudhof, T.C. Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science 323, 516–521 (2009).
Chen, X. et al. Three-dimensional structure of the complexin/SNARE complex. Neuron 33, 397–409 (2002).
Xue, M. et al. Distinct domains of complexin I differentially regulate neurotransmitter release. Nat. Struct. Mol. Biol. 14, 949–958 (2007).
Trimbuch, T. et al. Re-examining how complexin inhibits neurotransmitter release. eLife. 3, e02391 (2014).
Ma, C., Su, L., Seven, A.B., Xu, Y. & Rizo, J. Reconstitution of the vital functions of Munc18 and Munc13 in neurotransmitter release. Science 339, 421–425 (2013).
Rizo, J., Chen, X. & Arac, D. Unraveling the mechanisms of synaptotagmin and SNARE function in neurotransmitter release. Trends Cell Biol. 16, 339–350 (2006).
Bennett, M.K., Calakos, N. & Scheller, R.H. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science 257, 255–259 (1992).
Li, C. et al. Ca2+-dependent and -independent activities of neural and non-neural synaptotagmins. Nature 375, 594–599 (1995).
Gerona, R.R., Larsen, E.C., Kowalchyk, J.A. & Martin, T.F. The C terminus of SNAP25 is essential for Ca2+-dependent binding of synaptotagmin to SNARE complexes. J. Biol. Chem. 275, 6328–6336 (2000).
Chapman, E.R., Hanson, P.I., An, S. & Jahn, R. Ca2+ regulates the interaction between synaptotagmin and syntaxin 1. J. Biol. Chem. 270, 23667–23671 (1995).
Zhang, X., Kim-Miller, M.J., Fukuda, M., Kowalchyk, J.A. & Martin, T.F. Ca2+-dependent synaptotagmin binding to SNAP-25 is essential for Ca2+-triggered exocytosis. Neuron 34, 599–611 (2002).
Rickman, C. et al. Conserved prefusion protein assembly in regulated exocytosis. Mol. Biol. Cell 17, 283–294 (2006).
Dai, H., Shen, N., Arac, D. & Rizo, J. A quaternary SNARE-synaptotagmin-Ca2+-phospholipid complex in neurotransmitter release. J. Mol. Biol. 367, 848–863 (2007).
Lai, A.L., Huang, H., Herrick, D.Z., Epp, N. & Cafiso, D.S. Synaptotagmin 1 and SNAREs form a complex that is structurally heterogeneous. J. Mol. Biol. 405, 696–706 (2011).
Choi, U.B. et al. Single-molecule FRET–derived model of the synaptotagmin 1–SNARE fusion complex. Nat. Struct. Mol. Biol. 17, 318–324 (2010).
Zhou, A., Brewer, K.D. & Rizo, J. Analysis of SNARE complex/synaptotagmin-1 interactions by one-dimensional NMR spectroscopy. Biochemistry 52, 3446–3456 (2013).
Xu, J., Brewer, K.D., Perez-Castillejos, R. & Rizo, J. Subtle interplay between synaptotagmin and complexin binding to the SNARE complex. J. Mol. Biol. 425, 3461–3475 (2013).
Ruschak, A.M. & Kay, L.E. Methyl groups as probes of supra-molecular structure, dynamics and function. J. Biomol. NMR 46, 75–87 (2010).
Otting, G. Protein NMR using paramagnetic ions. Annu. Rev. Biophys. 39, 387–405 (2010).
Zhang, Y. & Cremer, P.S. Interactions between macromolecules and ions: the Hofmeister series. Curr. Opin. Chem. Biol. 10, 658–663 (2006).
Richens, J.L., Lunt, E.A., Sanger, D., McKenzie, G. & O′Shea, P. Avoiding nonspecific interactions in studies of the plasma proteome: practical solutions to prevention of nonspecific interactions for label-free detection of low-abundance plasma proteins. J. Proteome Res. 8, 5103–5110 (2009).
de la Cruz, L. et al. Binding of low molecular weight inhibitors promotes large conformational changes in the dengue virus NS2B–NS3 protease: fold analysis by pseudocontact shifts. J. Am. Chem. Soc. 133, 19205–19215 (2011).
Graham, B. et al. DOTA-amide lanthanide tag for reliable generation of pseudocontact shifts in protein NMR spectra. Bioconjug. Chem. 22, 2118–2125 (2011).
Schmitz, C. & Bonvin, A.M. Protein-protein HADDocking using exclusively pseudocontact shifts. J. Biomol. NMR 50, 263–266 (2011).
Shishmarev, D. & Otting, G. How reliable are pseudocontact shifts induced in proteins and ligands by mobile paramagnetic metal tags? A modelling study. J. Biomol. NMR 56, 203–216 (2013).
Camilloni, C., Robustelli, P., De, S.A., Cavalli, A. & Vendruscolo, M. Characterization of the conformational equilibrium between the two major substates of RNase A using NMR chemical shifts. J. Am. Chem. Soc. 134, 3968–3971 (2012).
DeSimone, A., Montalvao, R.W. & Vendruscolo, M. Determination of conformational equilibria in proteins using residual dipolar couplings. J. Chem. Theory Comput. 7, 4189–4195 (2011).
Araç, D., Murphy, T. & Rizo, J. Facile detection of protein-protein interactions by one-dimensional NMR spectroscopy. Biochemistry 42, 2774–2780 (2003).
Bai, J., Tucker, W.C. & Chapman, E.R. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat. Struct. Mol. Biol. 11, 36–44 (2004).
Radhakrishnan, A., Stein, A., Jahn, R. & Fasshauer, D. The Ca2+ affinity of synaptotagmin 1 is markedly increased by a specific interaction of its C2B domain with phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 284, 25749–25760 (2009).
Xu, J., Mashimo, T. & Sudhof, T.C. Synaptotagmin-1, -2, and -9: Ca2+ sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron 54, 567–581 (2007).
Xu, J., Pang, Z.P., Shin, O.H. & Sudhof, T.C. Synaptotagmin-1 functions as a Ca2+ sensor for spontaneous release. Nat. Neurosci. 12, 759–766 (2009).
Mittermaier, A.K. & Kay, L.E. Observing biological dynamics at atomic resolution using NMR. Trends Biochem. Sci. 34, 601–611 (2009).
Kim, J.Y. et al. Solution single-vesicle assay reveals PIP2-mediated sequential actions of synaptotagmin-1 on SNAREs. EMBO J. 31, 2144–2155 (2012).
Rickman, C. et al. Synaptotagmin interaction with the syntaxin/SNAP-25 dimer is mediated by an evolutionarily conserved motif and is sensitive to inositol hexakisphosphate. J. Biol. Chem. 279, 12574–12579 (2004).
Malsam, J. et al. Complexin arrests a pool of docked vesicles for fast Ca2+-dependent release. EMBO J. 31, 3270–3281 (2012).
Seven, A.B., Brewer, K.D., Shi, L., Jiang, Q.X. & Rizo, J. Prevalent mechanism of membrane bridging by synaptotagmin-1. Proc. Natl. Acad. Sci. USA 110, E3243–E3252 (2013).
Shin, O.H., Xu, J., Rizo, J. & Sudhof, T.C. Differential but convergent functions of Ca2+ binding to synaptotagmin-1 C2 domains mediate neurotransmitter release. Proc. Natl. Acad. Sci. USA 106, 16469–16474 (2009).
Lee, J., Guan, Z., Akbergenova, Y. & Littleton, J.T. Genetic analysis of synaptotagmin C2 domain specificity in regulating spontaneous and evoked neurotransmitter release. J. Neurosci. 33, 187–200 (2013).
Lynch, K.L. et al. Synaptotagmin C2A loop 2 mediates Ca2+-dependent SNARE interactions essential for Ca2+-triggered vesicle exocytosis. Mol. Biol. Cell 18, 4957–4968 (2007).
Delaglio, F. et al. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 (1995).
Johnson, B.A. & Blevins, R.A. NMR View: a computer-program for the visualization and analysis of NMR data. J. Biomol. NMR 4, 603–614 (1994).
Shao, X., Fernandez, I., Sudhof, T.C. & Rizo, J. Solution structures of the Ca2+-free and Ca2+-bound C2A domain of synaptotagmin I: does Ca2+ induce a conformational change? Biochemistry 37, 16106–16115 (1998).
Chen, X., Tang, J., Sudhof, T.C. & Rizo, J. Are neuronal SNARE proteins Ca2+ sensors? J. Mol. Biol. 347, 145–158 (2005).
Schmitz, C., Stanton-Cook, M.J., Su, X.C., Otting, G. & Huber, T. Numbat: an interactive software tool for fitting Deltachi-tensors to molecular coordinates using pseudocontact shifts. J. Biomol. NMR 41, 179–189 (2008).
Camilloni, C. & Vendruscolo, M. Statistical mechanics of the denatured state of a protein using replica-averaged metadynamics. J. Am. Chem. Soc. 136, 8982–8991 (2014).
Maximov, A., Pang, Z.P., Tervo, D.G. & Sudhof, T.C. Monitoring synaptic transmission in primary neuronal cultures using local extracellular stimulation. J. Neurosci. Methods 161, 75–87 (2007).
Pang, Z.P., Cao, P., Xu, W. & Sudhof, T.C. Calmodulin controls synaptic strength via presynaptic activation of calmodulin kinase II. J. Neurosci. 30, 4132–4142 (2010).
Bacaj, T. et al. Synaptotagmin-1 and synaptotagmin-7 trigger synchronous and asynchronous phases of neurotransmitter release. Neuron 80, 947–959 (2013).
Zhou, P. et al. Syntaxin-1 N-peptide and Habc-domain perform distinct essential functions in synaptic vesicle fusion. EMBO J. 32, 159–171 (2013).
Sharma, M. et al. CSPα knockout causes neurodegeneration by impairing SNAP-25 function. EMBO J. 31, 829–841 (2012).
Acknowledgements
We thank L. Kay for fruitful discussions and F. Peters (Max Planck Institute for Biophysical Chemistry) for generously providing a sample for Cys-Ph-TAHA–labeling. The DD2 console of one of the Agilent 600-MHz NMR spectrometers used for the research presented here was purchased with shared instrumentation grant S10RR026461 from the US National Institutes of Health (to M.K. Rosen; University of Texas Southwestern Medical Center). The authors acknowledge the Texas Advanced Computing Center at the University of Texas at Austin for providing high-performance computer resources that have contributed to the research results reported within this paper. This work was supported by Welch Foundation grant I-1304 (to J.R.), Australian Research Council (ARC) Discovery Grant DP150100383 (to B.G.), ARC Future Fellowship FT130100838 (to B.G.), Swiss National Science Foundation grant 200021_130263 (to D.H.) and US National Institutes of Health grants K99NS087086 (to T.B.) and NS040944 (to J.R.).
Author information
Authors and Affiliations
Contributions
K.D.B., T.B., J.D.S., A.Z., P.Z., N.B., J.X., A.B.S. and E.A.P. performed experiments and analyzed data. A.C., C.C., J.L. and R.V. performed computational analyses. D.H., A.M.J.J.B., D.R.T., M.V., B.G. and T.C.S. designed experiments and analyzed data. J.R. analyzed data and wrote the manuscript with input from all coauthors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Effects of Syt1 C2AB on the 1H-15N HSQC spectra of the SNARE complex.
(a) Diagram depicting the proteins involved in neurotransmitter release discussed in this study. The SNARE complex is depicted as a four-helix bundle with Synaptobrevin in red, Syntaxin-1 in yellow and SNAP-25 in green and blue. The N-terminal region of Syntaxin-1 is not shown for simplicity. The SNARE complex is shown partially formed and full zippering of the Synaptobrevin SNARE motif to complete the four-helix bundle is believed to be key for membrane fusion. Syt1 is shown in orange with the C2A and C2B domains labeled as A and B, respectively, while CpxI is shown in gray (central helix) and pink (accessory helix). The location of CpxI is based on the crystal structure of the CpxI(26-83)-SNARE complex1. The position of Syt1 is arbitrary and is the subject of this study. Only one molecule each of Syt1 and of CpxI are shown for simplicity but each SNARE complex is expected to have one molecule of Syt1 and one of CpxI bound. (b-d) 1H-15N TROSY HSQC spectra of 40 μM SNARE complex samples 2H,15N-labeled at the syntaxin-1 (b), synaptobrevin (c), SNAP-25 N-terminal (SNN) (d) and SNAP-25 C-terminal (SNC) (e) SNARE motifs in the absence (black contours) and presence (red contours) of 20 μM C2AB.
Supplementary Figure 2 Evaluations of PCSs induced by SC166Dy and SC41Dy.
(a-e) Leu,Val region of 1H-13C HMQC spectra of 30 μM 15N,2H-ILV-13CH3-labeled C2AB (a,d) or C2B (b,c,e) in the presence of SC166Dy (a-c) or SC41Dy (d,e) before (red contours) or after (black contours) removal of the tag by reduction. The concentration of SC166Dy or SC41Dy was 20 μM in (a,d) and 30 μM in (b,c,e). In (c), blue contours show a spectrum of 15N,2H-ILV-13CH3-C2B bound to SC166Dy in the presence of CpxI. Note that in panels (a,c) the contour levels were chosen to allow visualization of some of the PCSs and at the same time avoid the overcrowding observed at lower contour levels. Some of the cross-peaks from the red spectra are not observable at these contour levels due to the broadening caused by the lanthanide tag, but most cross-peaks are observable at lower contour levels and reveal PCSs that are parallel to those observed for C2B in panels (b,e). (f,g) Ile region of 1H-13C HMQC spectra of 30 μM SC166Dy (f) and SC41Dy (g) containing 15N,2H-ILV-13CH3-syntaxin-1 acquired after removal of the tag (black contours) or before removal of the tag in the absence (red contours) or presence (blue contours) of 30 μM C2B domain bearing the R398Q R399Q mutation (C2BRR).
Supplementary Figure 3 Comparing the SC166 and C2B166 tensors.
(a) Ribbon diagram of the C2B domain and isosurfaces representing regions with positive (blue) and negative (red) PCSs, contoured at ± 0.8 ppm with the C2B166 tensor. The values for Δχax and Δχrh (10−32 m3) are 32.0 and 18.4, respectively. (b) Ribbon diagram of the C2B domain and the SNARE complex with isosurfaces representing regions with positive (blue) and negative (red) PCSs, contoured at ± 0.8 ppm with the SC166 tensor. This is the same tensor illustrated in Fig. 2c but rotated to allow comparison with the C2B166 tensor shown in panel A. The position of C2B was derived by superimposing the centers of the SC166 and C2B166 tensors. (c) Correlation between experimental C2B PCSs caused by SC166Dy and PCSs calculated with the C2B166 tensor illustrated in panel (A). (d) Correlation between experimental C2B PCSs caused by SC166Dy and PCSs calculated with the SC166 tensor in the model resulting after superimposing the C2B166 and SC166 tensors (illustrated in panel b). (e) Correlation between experimental C2B PCSs caused by SC166Dy and PCSs calculated with the SC166 tensor in the model with C2B rotated, illustrated with C2B in orange in panel (f). (f) Ribbon diagram of the SNARE complex with isosurfaces representing regions with positive (blue) and negative (red) PCSs, contoured at ± 0.8 ppm with the SC166 tensor, showing the positions of C2B after superimposing the centers of the SC166 and C2B166 tensors (cyan ribbon) and after rotating C2B around the vertical axis (defined here by the negative lobes of the SC166 tensor) to make contact with the SNARE complex (orange ribbon). CpxI(26-83) is also shown (in pink) based on superimposing the models with the crystal structure of the CpxI(26-83)-SNARE complex (PDB code 1KIL) to illustrate that C2B would have steric clashes with CpxI(26-83) in the position of the orange ribbon. (g) Ribbon diagram of the SNARE complex with isosurfaces representing regions with positive (blue) and negative (red) PCSs, contoured at ± 0.8 ppm with the SC166 tensor, showing in orange the rotated position of C2B from panel (f) and in gray the position of C2B from the 166 manual model of Fig. 3e. In (a,b,f,g), the tensor center is indicated with a black sphere. Note that in (f) the rotated C2B (orange) is at the same distance from the tensor center as in the original model obtained by superimposing the centers of the C2B166 and SC166 tensors (cyan C2B), and that in (g) C2B from the 166 manual model (gray) is considerably closer to the tensor center than the rotated C2B (orange).
Supplementary Figure 4 Evaluation of selected models of the C2B–SNARE complex.
(a,c) Correlations between experimental C2B PCSs induced by SC166Dy and PCSs calculated with the SC166 tensor and the 166 HADDOCK model (a) or the 166 MD model (c). Correlation coefficients (r) and slopes (m) are indicated. (b,d) Representations of the 166 HADDOCK model (b) and 166 MD model (d) with the SNAREs and C2B shown as semi-transparent ribbons and the positive-negative lobes of the SC166 tensor represented by isosurfaces as in Fig. 2c. The C2B PCSs induced by SC166Dy are illustrated to visualize how well the positive-negative patterns match with the positive-negative lobes of the SC166 tensor. Amide hydrogens and methyl carbons are shown as spheres and color-coded according to the measured PCSs (dark blue, > 0.06 ppm; blue, 0.04 to 0.06 ppm; cyan, 0.02 to 0.04 ppm; pale cyan, 0.008 to 0.02 ppm; red, −0.04 to −0.06 ppm; salmon, −0.02 to −0.04 ppm; light pink, −0.008 to −0.02 ppm). Ca2+ ions are represented by yellow spheres.
Supplementary Figure 5 The 41 MD model.
Ribbon diagram of the 41 MD model with C2B shown in orange and Ca2+ ions represented by cyan spheres. Stick models show the side chains of basic (blue) and acidic (red) residues. Basic side chains from the polybasic strand and the concave side of C2B are labeled. N and C represent the N- and C-termini of the SNARE complex, respectively.
Supplementary Figure 6 Mutagenesis verifies the binding mode of the C2B–SNARE complex.
(a,b) Plots of normalized intensities of the SMRs in 1D 13C-edited 1H-NMR spectra of 3 μM WT or mutant 13C-C2AB as a function of SNARE complex concentration. C2AB mutants contained single substitutions in basic residues as indicated and color-coded. The data were acquired in 25 mM Tris (pH 7.4), 125 mM NaCl and 1 mM CaCl2, and were fitted to a single-site binding model2. (c,d) Bar diagrams illustrating the Kds derived from fitting the data of Figs. 5b,c to single-site binding models. The Kd values should be interpreted with caution because we did not use SNARE complex concentrations beyond 20 μM to minimize contributions from weaker binding modes and, as a consequence, the titrations were far from reaching saturation for the mutants with stronger effects on binding. Because of the uncertainty in the limiting intensities at infinite SNARE complex concentrations for the mutants that bind to the SNARE complex more weakly, this limiting value was forced to be 0.555 times the intensity at 0 SNARE complex concentration. The 0.555 factor was derived from averaging the ratios between intensities at 0 and infinite SNARE complex concentration in the fits obtained for WT C2AB. Bars show average Kds calculated from two independent experiments, and error bars show standard deviations.
Supplementary Figure 7 Overexpression levels of WT and double-mutant Syt1 used in rescue experiments.
(a) Sample Western blots illustrating the overexpression levels of the WT and double mutant Syt1 in the rescue experiments. (b) Quantification of the protein overexpression levels in three different independent experiments.
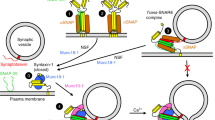
Supplementary Figure 8 Model of how Syt1 and the SNAREs trigger neurotransmitter release in an interplay with CpxI.
(a) Model of a primed state with a partially assembled SNARE complex where the C-terminus of the synaptobrevin SNARE motif remains flexible. CpxI(26-83) is shown according to the crystal structure of the CpxI(26-83)-SNARE complex (PDB accession code 1KIL). The position of Ca2+-free C2B corresponds to that of the 166 MD model. R398 and R399 at the bottom of C2B are shown as dark blue spheres and bound to the plasma membrane. (b) Model of a fused state with a fully assembled SNARE complex after Ca2+ influx, with C2B bound to the SNARE complex and to both membranes. Ca2+ ions are represented by yellow spheres. Key aspects of this model are: i) before Ca2+ influx, Syt1 binds to a partially assembled SNARE complex through the concave, basic side of the C2B domain and, in this primed state, the CpxI accessory helix (pink) repels the vesicle membrane, hindering membrane fusion; ii) Ca2+ binding to C2B induces binding to the vesicle membrane, forcing the inhibitory accessory helix to melt away; iii) simultaneous binding of R398-R399 of C2B to the plasma membrane and of the Ca2+-binding loops to the vesicle membrane forces the two membranes together which, together with full zippering of the SNARE complex, induces membrane fusion; iv) these actions of C2B may require re-orientation with respect to the SNARE complex, which would be facilitated by the dynamic nature of the C2B-SNARE complex interactions. This mechanism is consistent with the 166 MD model and 41 manual model, which we take as representatives of the preferred orientations defined by the two PCS datasets, as well as with many of the structures visited during our MD simulations. Hence, the proposed mechanism relies on the overall dynamic binding mode rather than on a biased choice of a particular model.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Notes 1–9 (PDF 1168 kb)
Rights and permissions
About this article
Cite this article
Brewer, K., Bacaj, T., Cavalli, A. et al. Dynamic binding mode of a Synaptotagmin-1–SNARE complex in solution. Nat Struct Mol Biol 22, 555–564 (2015). https://doi.org/10.1038/nsmb.3035
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3035
This article is cited by
-
The release of inhibition model reproduces kinetics and plasticity of neurotransmitter release in central synapses
Communications Biology (2023)
-
Vesicle trafficking and vesicle fusion: mechanisms, biological functions, and their implications for potential disease therapy
Molecular Biomedicine (2022)
-
Evaluation of the tert-butyl group as a probe for NMR studies of macromolecular complexes
Journal of Biomolecular NMR (2021)
-
Analysis of asymmetry in lipid and content mixing assays with reconstituted proteoliposomes containing the neuronal SNAREs
Scientific Reports (2020)
-
Resolving kinetic intermediates during the regulated assembly and disassembly of fusion pores
Nature Communications (2020)