Abstract
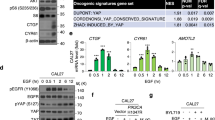
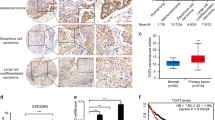
Malignant mesothelioma (MM) shows frequent inactivation of the neurofibromatosis type 2 (NF2) –tumor-suppressor gene. Recent studies have documented that the Hippo signaling pathway, a downstream cascade of Merlin (a product of NF2), has a key role in organ size control and carcinogenesis by regulating cell proliferation and apoptosis. We previously reported that MMs show overexpression of Yes-associated protein (YAP) transcriptional coactivator, the main downstream effector of the Hippo signaling pathway, which results from the inactivation of NF2, LATS2 and/or SAV1 genes (the latter two encoding core components of the mammalian Hippo pathway) or amplification of YAP itself. However, the detailed roles of YAP remain unclear, especially the target genes of YAP that enhance MM cell growth and survival. Here, we demonstrated that YAP-knockdown inhibited cell motility, invasion and anchorage-independent growth as well as cell proliferation of MM cells in vitro. We analyzed genes commonly regulated by YAP in three MM cell lines with constitutive YAP-activation, and found that the major subsets of YAP-upregulating genes encode cell cycle regulators. Among them, YAP directly induced the transcription of CCND1 and FOXM1, in cooperation with TEAD transcription factor. We also found that knockdown of CCND1 and FOXM1 suppressed MM cell proliferation, although the inhibitory effects were less evident than those of YAP knockdown. These results indicate that constitutive YAP activation in MM cells promotes cell cycle progression giving more aggressive phenotypes to MM cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Pass HI, Vogelzang N, Hahn S, Carbone M . Malignant pleural mesothelioma. Curr Probl Cancer 2004; 28: 93–174.
Yang H, Testa JR, Carbone M . Mesothelioma epidemiology, carcinogenesis, and pathogenesis. Curr Treat Options Oncol 2008; 9: 147–157.
Robinson BW, Lake RA . Advances in malignant mesothelioma. N Engl J Med 2005; 353: 1591–1603.
Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003; 21: 2636–2644.
Carbone M, Kratzke RA, Testa JR . The pathogenesis of mesothelioma. Semin Oncol 2002; 29: 2–17.
Sekido Y . Genomic abnormalities and signal transduction dysregulation in malignant mesothelioma cells. Cancer Sci 2010; 101: 1–6.
Illei PB, Ladanyi M, Rusch VW, Zakowski MF . The use of CDKN2A deletion as a diagnostic marker for malignant mesothelioma in body cavity effusions. Cancer 2003; 99: 51–56.
Bianchi AB, Mitsunaga SI, Cheng JQ, Klein WM, Jhanwar SC, Seizinger B et al. High frequency of inactivating mutations in the neurofibromatosis type 2 gene (NF2) in primary malignant mesotheliomas. Proc Natl Acad Sci USA 1995; 92: 10854–10858.
Sekido Y, Pass HI, Bader S, Mew DJ, Christman MF, Gazdar AF et al. Neurofibromatosis type 2 (NF2) gene is somatically mutated in mesothelioma but not in lung cancer. Cancer Res 1995; 55: 1227–1231.
Bott M, Brevet M, Taylor BS, Shimizu S, Ito T, Wang L et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet 2011; 43: 668–672.
Testa JR, Cheung M, Pei J, Below JE, Tan Y, Sementino E et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet 2011; 43: 1022–1025.
Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C et al. The tumour-suppressor genes NF2/Merlin and expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol 2006; 8: 27–36.
Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 2007; 130: 1120–1133.
Saucedo LJ, Edgar BA . Filling out the Hippo pathway. Nat Rev Mol Cell Biol 2007; 8: 613–621.
Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell 2010; 19: 27–38.
Murakami H, Mizuno T, Taniguchi T, Fujii M, Ishiguro F, Fukui T et al. LATS2 is a tumor suppressor gene of malignant mesothelioma. Cancer Res 2011; 71: 873–883.
Yokoyama T, Osada H, Murakami H, Tatematsu Y, Taniguchi T, Kondo Y et al. YAP1 is involved in mesothelioma development and negatively regulated by Merlin through phosphorylation. Carcinogenesis 2008; 29: 2139–2146.
Sekido Y . Inactivation of Merlin in malignant mesothelioma cells and the Hippo signaling cascade dysregulation. Pathol Int 2011; 61: 331–344.
Huang J, Wu S, Barrera J, Matthews K, Pan D . The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell 2005; 122: 421–434.
Tapon N, Harvey KF, Bell DW, Wahrer DC, Schiripo TA, Haber DA et al. Salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell 2002; 110: 467–478.
Cao X, Pfaff SL, Gage FH . YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes Dev 2008; 22: 3320–3334.
Nishioka N, Inoue K, Adachi K, Kiyonari H, Ota M, Ralston A et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell 2009; 16: 398–410.
Zhang X, Milton CC, Humbert PO, Harvey KF . Transcriptional output of the Salvador/Warts/Hippo pathway is controlled in distinct fashions in Drosophila melanogaster and mammalian cell lines. Cancer Res 2009; 69: 6033–6041.
Zhao B, Ye X, Yu J, Li L, Li W, Li S et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev 2008; 22: 1962–1971.
Hall CA, Wang R, Miao J, Oliva E, Shen X, Wheeler T et al. Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res 2010; 70: 8517–8525.
Muramatsu T, Imoto I, Matsui T, Kozaki K, Haruki S, Sudol M et al. YAP is a candidate oncogene for esophageal squamous cell carcinoma. Carcinogenesis 2011; 32: 389–398.
Wang Y, Dong Q, Zhang Q, Li Z, Wang E, Qiu X . Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci 2010; 101: 1279–1285.
Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT, Zender L et al. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer 2009; 115: 4576–4585.
Zhang X, George J, Deb S, Degoutin JL, Takano EA, Fox SB et al. The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene 2011; 30: 2810–2822.
Basu S, Totty NF, Irwin MS, Sudol M, Downward J . Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell 2003; 11: 11–23.
Lapi E, Di Agostino S, Donzelli S, Gal H, Domany E, Rechavi G et al. PML, YAP, and p73 are components of a proapoptotic autoregulatory feedback loop. Mol Cell 2008; 32: 803–814.
Levy D, Adamovich Y, Reuven N, Shaul Y . The Yes-associated protein 1 stabilizes p73 by preventing Itch-mediated ubiquitination of p73. Cell Death Differ 2007; 14: 743–751.
Anbanandam A, Albarado DC, Nguyen CT, Halder G, Gao X, Veeraraghavan S . Insights into transcription enhancer factor 1 (TEF-1) activity from the solution structure of the TEA domain. Proc Natl Acad Sci USA 2006; 103: 17225–17230.
Vassilev A, Kaneko KJ, Shu H, Zhao Y, DePamphilis ML . TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev 2001; 15: 1229–1241.
Xiao GH, Gallagher R, Shetler J, Skele K, Altomare DA, Pestell RG et al. The NF2 tumor suppressor gene product, merlin, inhibits cell proliferation and cell cycle progression by repressing cyclin D1 expression. Mol Cell Biol 2005; 25: 2384–2394.
Acknowledgements
We thank Ms Mika Yamamoto for her excellent technical assistance. This work was supported in part by a Special Coordination Fund for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science and Technology of Japan (H18-1-3-3-1), KAKENHI (18390245, 22300338), Grant-in-Aid for Third-Term Comprehensive Control Research for Cancer from the Ministry of Health, Labor and Welfare of Japan, the Takeda Science Foundation and the Kobayashi Foundation for Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Mizuno, T., Murakami, H., Fujii, M. et al. YAP induces malignant mesothelioma cell proliferation by upregulating transcription of cell cycle-promoting genes. Oncogene 31, 5117–5122 (2012). https://doi.org/10.1038/onc.2012.5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.5
Keywords
This article is cited by
-
Alternative Wnt-signaling axis leads to a break of oncogene-induced senescence
Cell Death & Disease (2024)
-
Carcinoma-associated fibroblast-derived lysyl oxidase-rich extracellular vesicles mediate collagen crosslinking and promote epithelial-mesenchymal transition via p-FAK/p-paxillin/YAP signaling
International Journal of Oral Science (2023)
-
Targeting CDK4 and CDK6 in cancer
Nature Reviews Cancer (2022)
-
Extracellular matrix and its therapeutic potential for cancer treatment
Signal Transduction and Targeted Therapy (2021)
-
Activation of AMPK inhibits Galectin-3-induced pulmonary artery smooth muscle cells proliferation by upregulating hippo signaling effector YAP
Molecular and Cellular Biochemistry (2021)