Abstract
Currently available therapeutic interventions for treatment-resistant depression, including switch, combination, and augmentation strategies, are less than ideal. Observations of mood elevation during vagus nerve stimulation (VNS) therapy for pharmacoresistant epilepsy suggested a role for VNS therapy in refractory major depression and prompted clinical investigation of this neurostimulation modality. The VNS Therapy System™ has been available for treatment of pharmacoresistant epilepsy since 1997 and was approved by the US Food and Drug Administration for treatment-resistant depression in July, 2005. The physiology of the vagus nerve, mechanics of the VNS Therapy System™, and efficacy and safety in pharmacoresistant epilepsy are reviewed. Promising results of VNS therapy for treatment-resistant depression have been forthcoming from both acute and long-term studies, evidenced in part by progressive improvements in depression rating scale scores during the 1st year of treatment with maintenance of response thereafter. VNS therapy is well tolerated in patients with either pharmacoresistant epilepsy or treatment-resistant depression. As in epilepsy, the mechanisms of VNS therapy of treatment-resistant depression are incompletely understood. However, evidence from neuroimaging and other studies suggests that VNS therapy acts via innervation of the nucleus tractus solitarius, with secondary projections to limbic and cortical structures that are involved in mood regulation, including brainstem regions that contain serotonergic (raphe nucleus) and noradrenergic (locus ceruleus) perikarya that project to the forebrain. Mechanisms that mediate the beneficial effects of VNS therapy for treatment-resistant depression remain obscure. Suggestions for future research directions are described.
Similar content being viewed by others
INTRODUCTION
Major depression is now recognized as a highly prevalent (Kessler et al, 2003), chronic, recurrent (Judd et al, 2000), and disabling biological disorder (Michaud et al, 2001) with high rates of morbidity and mortality. Indeed, major depression, which is projected to be the second leading cause of disability worldwide by the year 2020 (Michaud et al, 2001), is associated with high rates of mortality secondary to suicide and to the now well-established increased risk of death due to comorbid medical disorders, such as myocardial infarction and stroke (Carney and Freedland, 2003; Robinson, 2003). Considerable strides have been made over the past 2 decades in the development of safe and efficacious antidepressants. Although truly novel therapies with mechanisms other than monoamine neurotransmitter reuptake inhibition represent an active area of investigation, they are years away from being clinically available. Unfortunately, up to 50% of patients with depression do not achieve remission with currently available treatments in short-term (ie, 6–8 weeks), double-blind, clinical trials (Rush and Trivedi, 1995; Rush et al, 1998).
Therapeutic strategies that are employed for treatment-resistant depression are myriad and include multiple trials of high-dose antidepressants and varying combinations of antidepressants, augmenting agents, psychotherapy, and electroconvulsive therapy (ECT). A large body of evidence supports the acute efficacy of ECT in treatment-resistant depression. However memory loss and the need for repeated treatments to maintain efficacy preclude the use of ECT as a long-term treatment option (Rasmussen et al, 2002). Transcranial magnetic stimulation (Kauffmann et al, 2004; Schulze-Rauschenbach et al, 2005), stereotactic subcaudate tractotomy (Dalgleish et al, 2004; Malhi and Bartlett, 2000), limbic leukotomy (Montoya et al, 2002), and deep brain stimulation (Mayberg et al, 2005) are other options for treatment-resistant depression that are, to date, still experimental. One novel treatment modality has recently been approved by the US Food and Drug Administration (FDA). Vagus nerve stimulation (VNS) therapy, which is approved in the US and Europe for treatment of pharmacoresistant epilepsy, is now available in the US for treatment-resistant depression.
In this paper, we review the physiology of the vagus nerve and the mechanics of VNS technology. Data supporting the use of VNS therapy in pharmacoresistant epilepsy will be discussed briefly, as will the purported mechanisms underlying efficacy of VNS therapy in this patient population. Following that, we review evidence for the efficacy and safety of VNS therapy of treatment-resistant depression and examine findings from recently completed mechanism of action studies, most of which are preliminary and have been reported as abstracts, not peer-reviewed manuscripts. More detailed descriptions of mechanistic findings will be reported elsewhere. Our understanding of the mechanism of VNS therapy for treatment-resistant depression is in its infancy, and this paper concludes with a discussion of unmet research needs in the field.
THE VAGUS NERVE
The anatomy and physiology of the vagus nerve (cranial nerve X) is complex and until relatively recently, not well characterized. Named from the Latin word for ‘wandering,’ the vagus nerve exits the cranium through the jugular foramen, continuing distally through the jugular and nodose ganglia after which it travels between the jugular vein and carotid artery and from there to the larynx, esophagus, trachea, gastrointestinal organs, heart, and aorta. The vagus nerve consists of both efferent and afferent fibers. The parasympathetic efferent fibers provide autonomic regulation of the pharynx, larynx, esophagus, heart, aorta, and most gastrointestinal organs. Some of the efferent fibers in the right vagus nerve regulate heart rate. Somatomotor efferents to the vocal cords and other laryngeal striated muscles consist of highly myelinated, rapidly conducting components of the cervical vagus nerve, in contrast with the unmyelinated, slowly conducting parasympathetic efferents. Afferent fibers of the vagus nerve carry sensory information from the head, neck, abdomen, and thorax to the dorsal medullary complex, in particular the NTS. The perikarya of the vagal afferent fibers are located in the nodose ganglion. Approximately 80% of the nerve fibers in the cervical vagus nerve are afferent fibers (Figure 1).
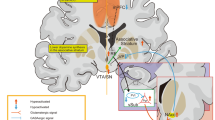
The left vagus nerve bifurcates on entering the medulla to innervate the NTS bilaterally, but only synapses ipsilaterally on the other nuclei of the dorsal medullary complex of the vagus. The NTS relays information to other brain regions via direct projections to the parabrachial nucleus (PBN), the cerebellum, the raphae, the periaqueductal gray (PAG), and the locus coeruleus, as well as ascending secondary projections to limbic, paralimbic, and cortical regions (Figures 2 and 3). Thus, the vagus nerve provides information to the brain in a bottom-up manner. The PBN is the structure that relays information from the NTS directly to the hypothalamus, thalamus, amygdala, and nucleus of the stria terminalis. Subsequently, the thalamus relays information to the insular, orbitofrontal, and prefrontal cortices, and other higher brain structures. Vagal projections to the locus ceruleus and raphe nuclei are important because they contain the perikarya of noradrenergic and serotonergic projections, respectively, that are implicated in the mechanism of action of traditional antidepressants (Lenox and Frazer, 2002). It is not known if vagus input to these individual regions are excitatory or inhibitory.
Limbic and cortical regions of the brain receive vagal afferents by multiple, overlapping routes. NTS=nucleus tractus solitarius; AMB=nucleus ambiguous; DMX=dorsal motor nucleus of the vagus; IML=intermediolateral column of spinal cord (symp pregang); VLM=ventrolateral medulla; Amyg=amygdala; INS=insular cortex; ILC=infralimbic cortex; BST=bed nucleus of stria terminalus; PBN=parabrachial nucleus; DR=dorsal raphe; LC=locus ceruleus; Thal=thalamus; Hypothal=hypothalamus. Green lines (tracts from the NTS); blue lines (tracts from the DR); red lines (tracts from the LC); light blue lines (tracts from the PBN). Courtesy of J Thomas Cunningham, University of Texas Health Science Center at San Antonio.
Projections from the NTS reach structures that are associated with the regulation of mood and emotion, seizure activity, anxiety, intestinal activity, satiety, and pain perception (Figures 2 and 3; see Berthoud and Neuhuber, 2000; George et al, 2004, 2000a, 2000b; Henry, 2002 for extensive reviews). The vagus nerve is now believed to be involved in nociception by relaying sensory information from the gastrointestinal and respiratory systems to higher brain regions as well as by mediating the affective-emotional response to pain (Berthoud and Neuhuber, 2000). Moreover, lamina I spinal neurons that mediate feelings of pain, temperature, and other physiological sensations project directly to the medulla, NTS, PBN, and PAG, and secondarily to the hypothalamus and limbic–cortical regions along a pathway that is similar to the afferent projections of the vagus nerve (Craig, 2002).
MECHANICS OF VNS
The VNS device consists of an implantable generator that is connected to electrodes which deliver low-frequency, chronic intermittent-pulsed electrical signals to the left cervical vagus nerve. The VNS Therapy System™ is commercially available for treatment of pharmacoresistant epilepsy and treatment-resistant depression (Cyberonics Inc., Houston, TX). The multiprogrammable pulse generator, which is roughly the size of a pocket watch, is implanted subcutaneously in the anterior chest wall during an outpatient surgical procedure similar to implantation of a cardiac pacemaker. Through a separate incision in the neck, the surgeon wraps the bipolar nerve-stimulating electrodes around the left cervical vagus nerve. Subsequently, the electrodes are connected to the implanted generator via a subcutaneous tunneling procedure (George et al, 2004; Matthews and Eljamel, 2003).
Clinicians are able to adjust stimulation parameters (ie, the ‘dose’) noninvasively with a telemetric wand that is linked to a hand-held personal digital assistant (Figure 4). Using the wand, the clinician can adjust the stimulation parameters, such as intensity, frequency, pulse width, and duty cycle (ie, duration of stimulation (‘on’ time) and interval between stimulation (‘off’ time)). The telemetric wand can be used to trigger a single duty cycle. Telemetric linkage also enables retrieval of data for further assessment of the effects of VNS therapy. Mechanical and electrical devices are built into the system as safety features to prevent high-frequency stimulation. In addition, patients are supplied with a magnet that can be held over the generator to temporarily suspend stimulation. Programmed stimulation resumes when the patient removes the magnet that, if needed, allows control over side effects (George et al, 2004; Matthews and Eljamel, 2003).
PHARMACORESISTANT EPILEPSY
The VNS Therapy System™ (Cyberonics Inc., Houston, TX) has been available in Europe since 1994 and in the United States since 1997 for the reduction of seizure frequency in patients with medically refractory, partial-onset seizures. There is extensive clinical experience with VNS therapy in this patient population. Over 30 000 patients with epilepsy have received VNS therapy for an accumulated experience of 79 000 patient years. The VNS Therapy System™ software enables a wide range of stimulation parameters, which consist of frequency (1–30 Hz), intensity (0–3.5 mA), duty cycle (7–60 s on: 12 s–180 min off), and pulse width (130–1000 μs). Typical initial stimulation parameters used in patients with epilepsy are 30 Hz, 0.1–1.0 mA, 30 s on : 5 min off, and 500 μs. Stimulation parameters are typically titrated after several months' time to higher current outputs (eg, 1.0–2.0 mA) and greater duty cycles (eg, 30 s on : 1.1 min off). Optimal stimulation parameters for VNS therapy in patients with pharmacoresistant epilepsy are not known, and individual patient characteristics guide the adjustments needed for a given clinical situation.
Efficacy and Safety in Epilepsy
The results of two prospective, randomized, double-blind studies of over 300 patients established the acute efficacy of VNS therapy of intractable seizures. A total of 313 patients were randomized to receive 12 or 14 weeks of high (30 Hz, 30 s on/5 min off; 500 μs pulse width) or low stimulation (1 Hz, 30 s on/90–180 min off; 130 μs pulse width) (Ben-Menachem et al, 1994; Handforth et al, 1998; The Vagus Nerve Stimulation Study Group, 1995). Reductions in seizure frequency were greater for the high stimulation groups (24.5% in the first study; 28% in the second study) than for the low stimulation group (6.1 and 15%, respectively; P⩽0.04 in both studies). VNS therapy was well tolerated. Adverse events were voice change, which was the most often reported adverse event, and dyspnea, cough, parasthesias, and infection or pain at the implantation site. Adverse effects on cognition, motor control, cardiac function, or gastrointestinal function did not occur more often in VNS therapy groups than in control groups.
Findings from open-label extensions of five short-term studies, including the two summarized above, demonstrate a sustained or improved level of seizure reduction and tolerance to adverse events (454 patients). Patients in the high stimulation groups continued to receive treatment with the same stimulation parameters, and patients treated with low stimulation were crossed over to high stimulation. Long-term VNS therapy resulted in a 35% reduction in seizure frequency at 1 year, 44.3% at 2 years, and 44.1% at 3 years. The proportion of patients with sustained seizure frequency reductions of 50% or greater was 23% at 3 months, 36.8% at 1 year, 43.2% at 2 years, and 42.7% at 3 years. Thus, it appears that the acute, 3-month response increases up to the 2nd year of treatment after which response rates tend to plateau (DeGiorgio et al, 2000; Morris et al, 1999; Salinsky et al, 1996). Moreover, response during acute treatment predicted maintenance of response at 1 year (Salinsky et al, 1996). Long-term treatment was well tolerated, with continuation rates of 96.7% at 1 year, 84.7% at 2 years, and 72.1% at 3 years (Morris et al, 1999).
Mechanism of Anticonvulsant Action
The mechanisms underlying the efficacy of VNS therapy for pharmacoresistant epilepsy are not known with precision, in part because the pathophysiology of epilepsy itself is incompletely understood. Indeed, one cannot speak of ‘one’ pathophysiology of epilepsy anymore than one can speak of ‘one’ pathophysiology of mood disorders. There are multiple causes of both disorders, each with subtypes that are characterized by distinct pathophysiology.
Seizures are characterized by synchronous firing of populations of neurons in the central nervous system, which is an observation that led to the hypothesis that VNS therapy converts synchronous cortical activity to desynchronous activity. Hammond et al (1992) tested this hypothesis in an open-label study of nine adults with intractable complex partial seizures. Acute VNS did not reduce focal spiking on the EEG during interictal periods. However, stimulation during the aura or shortly after the onset of an ictal episode was associated with reduced synchronous discharges (spiking) that, as speculated by the authors, prevented or interrupted the seizure.
Some have speculated that VNS therapy results in a global reduction in cortical excitability. Preliminary findings that were presented at the American Epilepsy Society 56th Annual meeting showed that VNS-implanted patients with epilepsy exhibited a higher resting motor threshold during activation of VNS compared to after the device was off for 30 min. The investigators interpreted these results to suggest that VNS may be associated with a reduction in motor cortex excitability (Dean et al, 2001). Nonetheless, the effects and implications of VNS therapy-related reductions in the synchronization of specific neural circuits are not known.
Technical challenges have hampered a systematic analysis of the mechanisms of VNS in animal models of seizures and epilepsy. The difficulty inherent in attaching electrodes to the very small vagus nerves of mice and rats is one limiting factor. In addition, the small size of these animals necessitates use of external generators that can provide short-term intermittent stimulation, but not chronic intermittent stimulation (George et al, 2004).
While systematic analysis of the mechanisms by which VNS therapy suppresses seizures has not been undertaken, several considerations support the hypothesis that seizures are controlled by regulating the efficacy of diffusely projecting afferents to the forebrain, such as the serotonergic or noradrenergic projections from the raphe and locus ceruleus, respectively. More specifically, VNS therapy is posited to control seizures arising from multiple cortical sites, which suggests that the sites at which VNS therapy controls neuronal excitability are widely distributed in the neocortex and archicortex. Furthermore, VNS therapy does desynchronize EEG recorded from electrodes widely distributed over the scalp, an effect that is consistent with regulating the efficacy of a population of diffusely projecting afferents. Widespread increases in blood flow in the hypothalamus, thalamus, insular cortex, and cerebellum and reduced blood flow in the hippocampus, amygdala, and posterior cingulate gyrus within 18 h of beginning VNS therapy in patients with partial epilepsy are consistent with the idea of widespread effects of VNS therapy on neuronal activity (Henry et al, 1998). Acute increases in thalamic blood flow during VNS therapy correlated with chronic reductions in seizure frequency (Henry et al, 1999). After chronic VNS therapy in the same cohort of patients, VNS therapy continued to increase blood flow in the same subcortical regions that had been activated acutely, but cortical regions showed much less activation on PET studies after chronic compared to acute VNS therapy (Henry et al, 2004). Similar to the imaging–efficacy correlations noted at acute VNS activation on PET, increased blood flow in the thalamus during chronic VNS therapy correlated with chronic reductions in seizure frequency. Bilateral thalamic activation as defined by increases in blood flow during VNS appears to be a marker of antiseizure effects, but the cellular and molecular mechanisms that underlie this correlation are unknown.
Given the powerful innervation of the NTS by vagal afferents, one important question is whether regulating the excitability of NTS neurons can affect seizure threshold. Interestingly, Walker et al (1999) examined the effects of intra-NTS microinjections of agonists or antagonists of the inhibitory neurotransmitter, γ-aminobutyric acid (GABA) and the excitatory neurotransmitter, L-glutamate, on seizure activity induced by bicuculline infusion into pyriform cortex. Administration of the GABA receptor agonist, muscimol, or the glutamate receptor antagonist, kynurenine, attenuated chemically induced seizures. Together these findings suggest that reducing the activity of neurons in NTS somehow suppresses limbic seizures and provides a valuable clue as to neural circuits mediating the antiseizure effects of VNS therapy. To date, there are no imaging data that correlate with these findings.
The antiseizure effects of reduced NTS neuronal activity raises the question of potential monosynaptic or polysynaptic targets that may mediate the anticonvulsant properties of VNS therapy. Two attractive candidates are the pars reticulata neurons of the substantia nigra (McNamara et al, 1984) and the raphe serotonergic neurons (Kovacs and Zoll, 1974; Siegel and Murphy, 1979). Suppressing the firing of zona reticulate substantia nigra neurons or increasing the firing of raphe neurons leads to elevated seizure thresholds, the former increasing focal thresholds for seizures evoked in olfactory bulb, amygdala, or entorhinal cortex (McNamara et al, 1984). How VNS therapy regulates the activity of NTS neurons and whether and how VNS therapy and manipulations of NTS regulate the activity of substantia nigra and raphe serotonergic neurons warrants future study.
TREATMENT-RESISTANT DEPRESSION
The rationale for studying VNS therapy in treatment-resistant depression was based on several different observations. Direct evidence supporting a role for VNS therapy in depression came from early observations of mood improvement in patients with epilepsy who participated in early VNS studies. Following these initial observations, patients with epilepsy were evaluated prospectively with standard depression symptom severity rating scales, which revealed that VNS therapy was associated with statistically significant improvements in mood that were not related to reductions in seizure frequency (Elger et al, 2000; Harden et al, 2000). In addition, several different lines of indirect evidence prompted further study of VNS therapy in depression. For example, PET imaging during VNS therapy of epilepsy demonstrated reductions in the metabolic activity of the amygdala, hippocampus, and cingulate gyrus (Henry et al, 1998), structures involved in regulating mood. These brain regions also are implicated during PET imaging in depressed patients who are being treated with antidepressants (Drevets et al, 2002; Mayberg et al, 2000; Nobler et al, 2001). Hippocampal involvement during VNS therapy also is consistent with the findings of studies showing increased expression of brain-derived neurotrophic factor (BDNF) in animal models of depression (Nibuya et al, 1995). The documented efficacy of anticonvulsants, such as carbamazepine, lamotrigine, valproate, and perhaps others, as mood stabilizers and/or antidepressants in bipolar disorder (Yatham, 2004) and the anticonvulsant properties of ECT (Kellinghaus et al, 2003; Lisanby et al, 2001) are concordant with the hypothesis that VNS therapy may be a useful therapeutic option for depression. Other findings that suggest a role for VNS therapy of depression include the effect of VNS therapy on brain regions associated with the norepinephrine (Krahl et al, 1998; Naritoku et al, 1995) and serotonin neural systems (Ben-Menachem et al, 1995), long thought to be important in the pathophysiology of depression. In addition, VNS has been shown to be as effective as ECT or desipramine in an animal model of depression (ie forced-swim test) (Krahl et al, 2004).
In 2001, the VNS Therapy™ System (Cyberonics Inc., Houston, TX) was approved for use in patients with treatment-resistant or treatment-intolerant major depressive episodes, including unipolar depression and bipolar disorder in Canada and the European Economic Area. To date, over 342 patients with treatment-resistant depression have received VNS therapy, with 777 patient years of cumulative clinical experience. Prior to the recent FDA approval of VNS therapy, the only approved medical device that was used in treatment-resistant depression was ECT. In accordance with the FDA Modernization Act of 1997, approval for treatments that address unmet medical needs are eligible for fast-track approval (eg antiretroviral agents for HIV infection). Consequently, FDA expedited approval for VNS therapy because of the lack of approved drug treatments and concerns about long-term efficacy and safety of ECT. Manufacturers of treatments approved via the fast-track process are required to conduct adequate follow-up studies to further characterize efficacy and safety. Such follow-up studies are ongoing for VNS therapy in patients with treatment-resistant depression.
As a prelude to the following review of VNS therapy for treatment-resistant depression, it is relevant to briefly compare and contrast FDA approval standards for drugs and devices. In their goal to expedite availability of new device technologies without compromising scientific integrity in the decision-making process, FDA has adopted the ‘least burdensome concept’ guidelines (CDRH, 2002), which is considered a successful means of addressing premarket issues that involves the smallest investment of time, effort, and resources on the part of the applicant and FDA. The least burdensome concept guidelines apply to all devices regulated by FDA.
Study design is an especially salient issue in the context of the FDA approval process. Approval of a new drug requires evidence of efficacy and safety from two positive, randomized, placebo-controlled trials. However, FDA requires a different standard of efficacy and safety evidence for medical devices in accordance with the least burdensome concept guidelines. Indeed, 55% of newly approved device applications are supported by data from nonrandomized clinical trials (Kahan, 2000). Difficulties in maintaining the study blind, which is a methodological challenge inherent in trials of medical devices, is a major concern for the design of device studies. The VNS therapy system requires an invasive surgical procedure to implant the device. Use of traditional placebo controls is neither applicable nor ethical under these circumstances, which lead to acceptance by the FDA of studies using sham controls, as in the VNS therapy trials (Demitrack, 2005). Therefore, the findings of medical device trials, including VNS therapy, should be interpreted with the understanding that, by design, they do not include a placebo arm. Many medical device trials involve before and after comparisons or comparison to historical controls.
Short-Term Efficacy
The acute effects of VNS therapy for treatment-resistant depression have been studied in a 10-week pilot study (N=60, Rush et al, 2000; Sackeim et al, 2001a) and in a larger, double-masked, sham-controlled, 10-week registration trial (N=235, Rush et al, 2005a). Of the 59 eligible patients in the pilot study, 30.5% (N=18) were responders (ie, ⩾50% reduction in baseline HAMD-28 total score), and 15.3% remitted (N=9; HAMD-28 total score ⩽10). Clinically meaningful improvement was gradual, with a mean time to response of 48.1 days. VNS therapy was well tolerated, and none of the 60 patients withdrew from the study because of adverse events.
The larger study was designed to compare VNS therapy with sham treatment in patients with treatment-resistant unipolar depression or depressed-phase, bipolar disorder (Rush et al, 2005a). This was a severely ill cohort, with a mean baseline HAMD-24 score of 29.2 and a mean duration of 49.1 months for the current episode. All patients were maintained for at least 4 weeks on a stable medication regimen before the preimplantation baseline assessment, and medications were not changed for the duration of the 12-week study. There were 112 patients in the treatment group, and 110 patients served as sham controls. The VNS therapy device was activated in the treatment group following a 2-week, single-blind recovery period after implantation. Stimulation parameters were adjusted within predetermined levels until the 3rd week postactivation when parameters were fixed for the remaining 8 weeks of the study. Median stimulation parameters at end point were 0.75 mA (intensity), 20 Hz (frequency), 500 μs (pulse width), 30 s (time on) : 5 min (time off). Although VNS therapy was very well tolerated, short-term efficacy was not demonstrated. Response rates, defined as ⩾50% reduction in baseline HAMD-24, for the active treatment and sham control groups were 15.2 and 10.0%, respectively (P=0.251; LOCF).
Long-Term Efficacy
Evidence suggesting progressive improvements in seizure control in patients with pharmacoresistant epilepsy provided the rationale for long-term study of VNS therapy for treatment-resistant depression. The 59 patients who completed the short-term pilot study (Rush et al, 2000; Sackeim et al, 2001a) and met response criteria at 3 months (early responders) or 1 year (late responders) were followed for a total of 2 years (Nahas et al, 2005). The 3-month response rates of 30.5% increased to 44.1% at 1 year and remained at that level (42.4%) at the 2-year time point (Nahas et al, 2005). Sackeim and colleagues presented data from this cohort at the 43rd Annual Meeting of the American College of Neuropsychopharmacology showing that when maintenance of response was considered, 55.6% of early responders and 78.6% of late responders continued to be responders at 2 years (Sackeim et al, 2004). Rates of remission in a subset of 30 patients, defined as ⩽10 on the HAMD-28, increased from 17% at 3 months (five of 30 patients) to 29% at 1 year (eight of 28 patients; P=0.045) (Marangell et al, 2002).
One-year naturalistic follow-up of 205 patients who completed the short-term registration trial (Rush et al, 2005a) revealed a similar pattern of later, but sustained response (Rush et al, 2005b). Patients in the active treatment group continued on VNS therapy for an additional 9 months, for a total of 12 months. Patients randomized to the sham control group in the short-term study (Rush et al, 2005a), were crossed over to a 12-month course of active VNS therapy (Rush et al, 2005b). In addition to VNS therapy, all patients continued to receive treatment as usual (TAU). At LOCF end point, the response rate was 27.2, and 15.8% of patients remitted. Rates of response and remission doubled between 3 and 12 months of treatment (P<0.005), indicating progressive clinical improvement after the initial 3 months of VNS therapy. Similar results were noted for the secondary clinical end points. Although direct, comparative studies are not available, the long-term benefits of VNS therapy compare favorably to ECT, in which a substantial majority of patients relapse within 6 months of achieving remission (Prudic et al, 2004; Sackeim et al, 2001b).
Data from the 205 patients who completed the 12-month naturalistic study (Rush et al, 2005b) were compared with a matched control group of 124 patients with treatment-resistant depression who received only TAU (George et al, 2005). The primary outcome measure was the difference in Inventory of Depressive Symptomatology Self-Report scores (IDS-SR30) per month between the VNS therapy plus TAU and the TAU groups. At end point, VNS therapy plus TAU was associated with a mean improvement on the IDS-SR30 score of 9.3 points, which represented a significantly greater improvement than TAU (4.2-point improvement; P<0.001). Differences in LOCF response rates between VNS therapy plus TAU (19.6%) and TAU (12.1%) did not achieve statistical significance (P=0.108). In contrast, LOCF remission rates were significantly greater in the VNS therapy plus TAU group (13.2%) compared to TAU (3.2%; P=0.007). Although these results are promising, extrapolation of the 1-year findings to clinical practice may be limited because the TAU group was not randomized and the TAU therapies were not restricted in either group after the first 3 months.
Mechanism of Action in Treatment-Resistant Depression
As with epilepsy, the mechanisms by which VNS therapy may benefit treatment-resistant depression are presently unclear. Different methodological approaches have been employed in an effort to better understand the mechanism of action of VNS therapy, such as mapping neural substrates and identifying changes in neurotransmitter systems during VNS therapy in patients with treatment-resistant depression.
Mapping neural substrates
Expanding on earlier findings (Naritoku et al, 1995, Kling et al (2003) measured expression of the protein encoded by the immediate early gene c-fos, which is a marker of neuronal activity, in rats exposed to short-term VNS or sham conditions. Their preliminary findings were presented at the 58th Annual Scientific Convention of the Society of Biological Psychiatry (Kling et al, 2003). Compared to control animals, VNS resulted in markedly increased c-fos expression in forebrain (lateral hypothalamus, paraventricular nuclei, CA3 hippocampal fields, and neocortex) and brain stem regions (NTS, nucleus raphe magnus, PBN, A7 area, locus ceruleus, and periaqueductal gray). These findings support the idea that VNS therapy acts directly by stimulating brain stem structures and indirectly by regulating the activity of neurons in limbic and cortical regions involved in mood regulation (Figures 2 and 3).
Functional neuroimaging studies in patients with treatment-resistant depression using SPECT, PET, or functional magnetic resonance imaging (fMRI) have been conducted to identify discrete brain regions that are affected by VNS therapy. This is an emerging literature that, at present, is difficult to interpret (Chae et al, 2003). The heterogeneity in imaging methods, small sample sizes, assorted diagnoses (eg unipolar major depression; bipolar disorder), varying types of antidepressant therapies, and different timeframes during which scans were obtained preclude definitive conclusions about this literature. In addition, none of the imaging studies have yet been replicated. Some findings appear to be similar to VNS therapy of pharmacoresistant epilepsy, while others demonstrate functional changes that may be unique to VNS therapy in patients with treatment-resistant depression, a subset of which are comparable to findings of antidepressant studies. Despite these limitations, the findings of recent imaging studies conducted after acute or during long-term VNS therapy in patients with treatment-resistant depression represent an important first step in elucidating the mechanisms of VNS therapy. The effects on medial temporal structures are of particular interest, given that such structures are theoretically important to both epilepsy and depression and have been implicated in the c-fos work in rats (Kling et al, 2003; Naritoku et al, 1995).
SPECT imaging studies: Findings from two SPECT imaging studies of patients with treatment-resistant depression have been reported. In their presentation of preliminary data from 11 patients with treatment-resistant depression at the 40th Annual Meeting of the ACNP, Devous and colleagues concluded that, compared with normal controls, a 10-week course of VNS therapy was associated with resolution of some of the regional cerebral blood flow (rCBF) abnormalities in limbic and cortical structures (eg insula, dorsolateral prefrontal cortex (DLPFC), temporal cortex) that are associated with depression. Of note, thalamic rCBF after 10 weeks was increased compared to controls (Devous et al, 2002). Two patients were responders and five were partial responders after 10 weeks of VNS therapy. Medial temporal findings of interest include decreased hippocampal rCBF at baseline in patients compared to controls and a correlation between HAMD scores and increased medial temporal cortex rCBF in patients after 10 weeks of VNS therapy. Changes seen in the anterior cingulate were inversely correlated with 6-month HAMD scores (n=11) (Devous et al, 2002).
Zobel et al (2005) reported the findings of SPECT imaging in 12 patients with treatment-resistant depression at baseline and after 4 weeks of VNS therapy. All patients were maintained on their antidepressant regimens during the study. Compared to baseline, a 4-week course of VNS therapy resulted in increased rCBF in the left middle frontal gyrus (BA 46) and reduced rCBF in the hippocampus/amygdala, left caudate, dorsal brainstem, and other areas via statistical parametric mapping (SPM) analyses. Region of interest (ROI) analyses demonstrated significant reductions in the right DLPFC, left subgenual cingulate, bilateral ventral anterior cingulate, right dorsal anterior cingulate, bilateral amygdala, left hippocampus, right thalamus, left caudate, and the brainstem. Changes in the right hippocampus trended toward significance. Medial temporal findings in this study included reduced rCBF in the amygdala and left hippocampus by both the SPM and ROI analyses at 4 weeks compared to baseline.
PET imaging studies: Results of 18F-fluorodeoxyglucose (FDG) PET imaging studies from two different centers have been presented. Conway and colleagues reported preliminary PET findings at the 57th Annual Scientific Convention of the Society for Biological Psychiatry from seven patients with treatment-resistant depression who were scanned prior to VNS activation and again after 12 weeks of VNS therapy. They observed increased metabolic activity in the orbitofrontal cortex, amygdala, parahippocampal gyrus, insula, and cingulate gyrus. In this study, the medial temporal findings consisted of increased metabolic activity in the left parahippocampal gyrus. Unlike the SPECT study by Zobel et al (2005), reductions in activation of these regions were not noted (Conway et al, 2002).
In contrast, findings of widespread reductions in midbrain glucose metabolism were observed by another group of investigators in a preliminary study of chronic VNS therapy of treatment-resistant depression that was presented at the 58th Annual Scientific Convention of the Society of Biological Psychiatry (Hagen et al, 2003; Sheikh et al, 2003). After a 1-year course of VNS therapy in eight patients, decreased metabolism compared to baseline was noted in the substantia nigra, ventral tegmentum, hypothalamus, middle and inferior frontal gyrus, insula/claustrum, and superior temporal gyrus. Increased glucose metabolism compared to baseline occurred in the cerebellum, precuneus-BA7, fusiform gyrus-BA37, and medial frontal-BA6 (Sheikh et al, 2003). In this same cohort, five patients exhibiting improvement at 1 year showed greater increases in metabolism in the occipital and temporal lobes, middle frontal gyrus, and inferior frontal gyrus and greater reductions in the midbrain (substantia nigra, ventral tegmentum), cerebellum, posterior cingulate, medial frontal/pregenual cingulate, and hippocampus compared to three patients who did not improve (Hagen et al, 2003). Relevant medial temporal findings include decreased FDG metabolism at 1 year in the left hippocampus of patients whose depression improved during VNS therapy vs patients who did not respond.
BOLD fMRI studies: A feasibility study of blood oxygenation level (BOLD) fMRI in nine patients with treatment-resistant depression who were chronically treated with VNS and antidepressants also revealed changes in brain function (Bohning et al, 2001). The BOLD fMRI signal is thought to be analogous to rCBF changes seen with SPECT and PET (Casey, 2000), but this is not entirely certain. The BOLD fMRI changes in these patients following a stimulation cycle of 13 s on and 103 s off consisted of bilateral activation of the orbitofrontal and parieto-occipital cortex and activation of the left temporal cortex (as reported by Devous et al, 2002), the left amygdala (as reported by Conway et al, 2002), and the hypothalamus (in contrast to findings of Sheikh et al, 2003).
Summary of medial temporal imaging findings: Despite the heterogeneity of methods and the overall lack of statistical power, it is nonetheless striking that each imaging study revealed changes in medial temporal structures. Changes were observed in the amygdala in some studies and in the hippocampus or parahippocampus in others. In addition, medial temporal changes were sometimes bilateral, and in some studies, changes were limited to the left medial temporal regions. Moreover, some, but not all, of the studies showed decreases in medial temporal rCBF or FDG metabolism (over varying time points). Clearly, future studies are needed to better characterize changes in these brain regions. A study with a sufficiently large patient sample that provides adequate power should help determine if changes are bilateral or lateralized and which temporal structures are involved and which are not.
Identifying neurotransmitter systems
Studies have been conducted recently to identify the effects of VNS therapy on neurotransmitter systems. In one study, 21 patients with treatment-resistant depression who were participating in a clinical trial of VNS therapy consented to undergo cerebrospinal fluid (CSF) collections at postimplantation (baseline) and again after 12 and 24 weeks of treatment (Carpenter et al, 2004). Ten of the patients served as sham subjects for 12 weeks, after which they were crossed over to active VNS therapy. The CSF samples were assayed for concentrations of norepinephrine, 5-hydroxyindoleacetic acid (5-HIAA), homovanillic acid (HVA), 3-methoxy-4-hydroxyphenylglycol, and GABA. Compared to sham conditions, active VNS therapy was associated with significantly elevated CSF concentrations of HVA, but no changes in the other substrates. An increased ratio of HVA : 5-HIAA levels correlated with clinical improvement with VNS therapy.
The SSRIs are believed to exert their antidepressant effect in a two-stage process: first, SSRIs increase 5HT concentrations by blocking 5HT reuptake, increasing the occupancy of the 5HT1A autoreceptors by 5HT, decreasing the firing rate of the serotonin neurons, and inhibiting serotonin release; and second, by eventually downregulating the autoreceptors and promoting release of serotonin from the presynaptic neuron (Lenox and Frazer, 2002). Serotonin is densely concentrated in dorsal raphe nucleus neurons in the midbrain. The effects of VNS on the firing rates of dorsal raphe serotonergic neurons in rats exposed to acute and chronic VNS or sham stimulation were described in a preliminary report presented at the Society for Neuroscience Annual Meeting (Debonnel and Dorr, 2004). Unlike SSRIs, VNS was not associated with an initial reduction in the firing rates of serotonergic neurons. Rather, raphe neuron firing rates progressively increased over 2 weeks (Debonnel and Dorr, 2004), which is consistent with the progressive increase in antidepressant response observed in clinical studies of VNS therapy and with antiseizure effects of serotonin (Clinckers et al, 2004). Also of interest was the finding that VNS therapy did not result in downregulation of the 5HT1A autoreceptors, suggesting that VNS therapy alters serotonin availability by a mechanism that is distinct from the SSRIs.
UNMET RESEARCH NEEDS
One key limitation to the general study of treatment-resistant depression is the lack of a validated and universally accepted definition of treatment resistance. Currently, treatment history and the duration, recurrence, and severity of depressive episodes form the boundaries of ‘treatment resistance.’ In the not too distant future, it is reasonable to expect that subtypes of treatment resistance will be defined in neurobiological terms in the same way that various causes of refractory hypertension, such as pheochromocytoma, have been identified. Expression of genetic polymorphisms in the serotonin transporter, MRI evidence of white matter hyperintensities, hippocampal volume reduction, regional imaging markers (eg subgenual cingulate and thalamic hyperactivity), and neuroendocrine data (eg salivary cortisol levels) represent only a few of the possible parameters on which to base a biological definition of treatment-resistant depression.
Our understanding of the neurobiological mechanisms of VNS therapy is just beginning to take shape. This novel technology offers a new treatment modality for the most seriously ill of the sizable depressed patient population and provides a unique opportunity to further our understanding of the pathophysiology of depression. As the field moves forward, a general research model is needed to meet the dual goals of elucidating the mechanism of action of VNS therapy and improving clinical outcomes. Preclinical research should initially focus on a comprehensive description of the brain areas affected by VNS (ie its functional neuroanatomy) and the effects of different stimulation parameters. Clinical studies should build on the findings of work in animal models and further investigate optimal stimulation parameters (ie dosing studies), the role of VNS therapy in different patient populations, and the identification of biomarkers of risk and response. Some specific preclinical and clinical research questions related to mechanism of action/pathophysiology and clinical efficacy include:
Mechanisms/Pathophysiology
-
What are the cellular and molecular mechanisms by which VNS elevates seizure threshold in animal models of epilepsy?
-
In the absence of an animal model for treatment-resistant depression, is VNS effective in animal models of depression? Laboratory animal studies, including those in non-human primates, should take full advantage of contemporary methods to determine the effects of VNS on a variety of neurotransmitter systems and on synaptic plasticity.
-
Does chronic VNS in laboratory animals produce effects that are similar to the SSRIs, selective norepinephrine reuptake inhibitors, ECT, and other antidepressant treatments, such as changes in BDNF and increased hippocampal neurogenesis?
-
How do the effects of acute and chronic VNS therapy of treatment-resistant depression differ? Does chronic VNS therapy induce synaptic plasticity?
Clinical Outcome
-
What are the optimal stimulation parameters of VNS for elevation of seizure threshold in animal models of epilepsy?
-
Is it possible to identify surrogate markers of VNS (eg, fMRI, PET, neuroendocrine data) that correlate with maximally elevated seizure threshold in animal models of epilepsy?
-
Are stimulation parameters that are optimal for the treatment of epilepsy also optimal for the treatment of depression?
-
What are the optimal stimulation parameters of VNS therapy for response and remission of treatment-resistant depression?
-
How do the stimulation parameters and the time course of imaging findings relate to the observed delays in therapeutic response?
-
What is the relationship between stimulation parameters and regional brain changes in responders vs nonresponders?
-
Considering the fact that all of the VNS depression treatment trials were conducted in patients who were maintained on existing psychopharmacologic regimens, to what extent does VNS therapy augment the behavioral and neurobiological effects of currently available antidepressants and anticonvulsants?
-
Can predictors of response to VNS therapy in depressed patients be identified by functional imaging or genetic studies?
-
Would modifications in stimulation parameters, such as higher frequencies or longer pulse widths, target different brain regions and possibly uncover biological markers for response or nonresponse in treatment-resistant depression?
The need for hypothesis-driven clinical studies specifically designed to identify stimulation parameters during VNS therapy of treatment-resistant depression that optimize remission rates remains a high priority. Ideally, stimulation parameters should first be identified in animal models; such studies are ongoing. Once optimal stimulation parameters are identified, they should be correlated with changes on functional imaging scans. BOLD fMRI has been used to determine if there is a dose–response of different stimulation parameters during VNS therapy of treatment-resistant depression (Lomarev et al, 2002; Mu et al, 2004). Thus far, a pulse width of 250 μs results in the same degree of global activation as a 500 μs pulse width. Moreover, a pulse width of 130 μs is not sufficient for immediate activation of some brain regions (Mu et al, 2004). A similar study was conducted to determine the effects of different stimulation frequencies on brain activation. A higher frequency of stimulation (ie 20 Hz) resulted in activation of more brain regions than the lower frequency of 5 Hz (Lomarev et al, 2002). Dose–response studies of other stimulation parameters will undoubtedly provide additional information to optimize VNS therapy.
There are many other priority items on the clinical research agenda for treatment-resistant depression. Predictors of response to VNS therapy and other strategies for treatment-refractory depression would represent a significant advance in clinical care. Clues from the relatively few clinical studies have provided some promising leads, such as an association between the changes in the ratio of CSF HVA : 5-HIAA concentrations during VNS therapy (Carpenter et al, 2004) and PET changes in ventral brain regions (Hagen et al, 2003).
Conclusions
VNS therapy appears to be a valuable addition to existing treatments for patients with pharmacoresistant epilepsy. The available data, taken together, suggests that VNS therapy also is a promising and well-tolerated intervention that is effective in a subset of patients with treatment-resistant depression. Evidence from clinical trials of VNS therapy in treatment-resistant depression is growing. As data accumulate, the role of VNS therapy in the depression treatment armamentarium will be better defined. A thorough understanding of the efficacy of new treatments is never completely clear when they are first approved, and VNS therapy is no exception. Dosing trials of VNS therapy in treatment-resistant depression are ongoing, and the findings of these studies in conjunction with the cumulative experience by clinicians will inform future therapeutic choices.
In addition to representing a novel therapeutic modality, VNS therapy is a research tool that offers the hope of better understanding and potentially treating a variety of brain diseases. At the current time, the mechanisms underlying its efficacy for treatment-resistant depression remain incompletely understood. One appealing explanation is that the effects of chronic VNS therapy are mediated by the known direct and secondary projections of the vagus nerve to brain regions involved in the regulation of mood. More specifically, the effects of VNS therapy on noradrenergic neurons in the locus ceruleus and serotonergic neurons in the raphe nucleus are one likely mechanism of action. Rosenbaum and Heninger (2000) speculated that the latency of response in patients with epilepsy or treatment-resistant depression suggests that VNS therapy may trigger a process of neural adaptation rather than directly targeting the specific pathophysiological deficits of the disorder. The results of ongoing, hypothesis-driven clinical and imaging studies will be pivotal to increasing our understanding of the mechanisms of action of VNS therapy of treatment-resistant depression.
References
Ben-Menachem E, Hamberger A, Hedner T, Hammond EJ, Uthman BM, Slater J et al (1995). Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res 20: 221–227.
Ben-Menachem E, Manon-Espaillat R, Ristanovic R, Wilder BJ, Stefan H, Mirza W et al (1994). Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First International Vagus Nerve Stimulation Study Group. Epilepsia 35: 616–626.
Berthoud H-R, Neuhuber WL (2000). Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 85: 1–17.
Bohning DE, Lomarev MP, Denslow S, Nahas Z, Shastri A, George MS (2001). Feasibility of vagus nerve stimulation-synchronized blood oxygenation level-dependent functional MRI. Invest Radiol 36: 470–479.
Carney RM, Freedland KE (2003). Depression, mortality, and medical morbidity in patients with coronary heart disease. Biol Psychiatr 54: 241–247.
Carpenter LL, Moreno FA, Kling MA, Anderson GM, Renegold WT, Labiner DM et al (2004). Effect of vagus nerve stimulation on cerebrospinal fluid monoamine metabolites, norepinephrine, and gamma-aminobutyric acid concentrations in depressed patients. Biol Psychiatr 56: 418–426.
Casey KL (2000). Imaging pain. International Association for the Study of Pain 8 http://www.iasp-pain.org/PCU00-4.html (Accessed September 8, 2005).
CDRH (Center for Devices and Radiological Health) (2002). The least burdensome provisions of the FDA modernization act of 1997: concept and principles; final guidance for FDA and industry. October 4, 2002; available at: http://www.fda.gov/cdrh/ode/guidance/1332.pdf (Accessed January 17, 2006).
Chae J-H, Nahas Z, Lomarev M, Denslow S, Lorberbaum JP, Bohning DE et al (2003). A review of functional neuroimaging studies of vagus nerve stimulation (VNS). J Psychiatr Res 37: 443–455.
Clinckers R, Smolders I, Meurs A, Ebinger G, Michotte Y (2004). Anticonvulsant action of hippocampal dopamine and serotonin is independently mediated by D and 5-HT receptors. J Neurochem 89: 834–843.
Conway CR, Chibnall JT, Fletcher JW, Filla-Taylor J, Grossberg GT, Li X et al (2002). Three months of vagus nerve stimulation is associated with increased limbic and paralimbic activity (FDG PET) in treatment-resistant depressed subjects. Poster presented at the 57th Annual Scientific Convention of the Society of Biological Psychiatry. Philadelphia, PA, May 16–18, 2002.
Craig AD (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3: 655–666.
Dalgleish T, Yiend J, Bramham J, Teasdale JD, Ogilvie AD, Malhi G et al (2004). Neuropsychological processing associated with recovery from depression after stereotactic subcaudate tractotomy. Am J Psychiatr 161: 1913–1916.
Dean AC, Wu AT, Burgat FT, Labar DT (2001). Motor cortex excitability in epilepsy patients treated with vagus nerve stimulation. Presented at the American Epilepsy Society 55th Annual Meeting. Philadelphia, PA, November 30–December 5, 2001.
Debonnel G, Dorr AE (2004). Effect of vagus nerve stimulation (VNS) on dorsal raphe serotonergic neurons: an electrophysiological study in the rat. Poster presented at the Society for Neuroscience Annual Meeting. San Diego, CA, October 23–27, 2004.
DeGiorgio CM, Schachter SC, Handforth A, Salinsky M, Thompson J, Uthman B et al (2000). Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia 41: 1195–1200.
Demitrack MA (2005). Examining the safety and effectiveness of transcranial magnetic stimulation for depression. Psychiatric Ann 35: 120–128.
Devous MD, Husain M, Harris TS, Rush AJ (2002). Effects of VNS on regional cerebral blood flow in depressed subjects. Poster presented at the 42nd Annual New Clinical Drug Evaluation Unit Meeting. Boca Raton, FL, June 10–13, 2002.
Drevets WC, Bogers W, Raichle ME (2002). Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur Neuropsychopharmacol 12: 527–544.
Elger G, Hoppe C, Falkai P, Rush AJ, Elger CE (2000). Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res 42: 203–210.
George MS, Nahas Z, Bohning DE, Mu Q, Kozel FA, Borckhardt J et al (2004). Mechanisms of action of vagus nerve stimulation (VNS). Clin Neurosci Res 4: 71–79.
George MS, Rush AJ, Marangell LB, Sackeim HA, Brannan SK, Davis SM et al (2005). A one-year comparison of vagus nerve stimulation with treatment as usual for treatment-resistant depression. Biol Psychiatr 58: 364–373.
George MS, Sackeim HA, Marangell LB, Husain MM, Nahas Z, Lisanby SH et al (2000a). Vagus nerve stimulation. A potential therapy for resistant depression? Psychiatr Clin North Am 23: 757–783.
George MS, Sackeim HA, Rush AJ, Marangell LB, Nahas Z, Husain MM et al (2000b). Vagus nerve stimulation: A new tool for brain research and therapy. Biol Psychiatr 47: 287–295.
Hagen MC, Sheikh S, Adson D, Rittberg B, Abuzzahab FS, Lee JT et al (2003). Metabolic changes in treatment-resistant depression responsive to VNS therapy. Poster presented at the 58th Annual Scientific Convention of the Society of Biological Psychiatry. San Francisco, CA, May 15–17, 2003.
Hammond EJ, Uthman BM, Reid SA, Wilder BJ (1992). Electrophysiological studies of cervical vagus nerve stimulation in humans: I. EEG effects. Epilepsia 33: 1013–1020.
Handforth A, DeGiorgio CM, Schachter SC, Uthman BM, Naritoku DK, Tecoma ES et al (1998). Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology 51: 48–55.
Harden CL, Pulver MC, Ravdin LD, Nikolov B, Halper JP, Labar DR (2000). A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav 1: 93–99.
Henry TR (2002). Therapeutic mechanisms of vagus nerve stimulation. Neurology 59 (Suppl 4): S3–S14.
Henry TR, Bakay RAE, Pennell PB, Epstein CM, Votaw JR (2004). Brain blood flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: II. Prolonged effects at high and low levels of stimulation. Epilepsia 45: 1064–1070.
Henry TR, Bakay RAE, Votaw JR, Pennell PB, Epstein CM, Faber TL et al (1998). Brain blood flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: I. Acute effects at high and low levels of stimulation. Epilepsia 39: 983–990.
Henry TR, Votaw JR, Pennell PB, Epstein CM, Bakay RAE, Faber TL et al (1999). Acute blood flow changes and efficacy of vagus nerve stimulation in partial epilepsy. Neurology 52: 1166–1173.
Judd LL, Paulus MJ, Schettler PJ, Akiskal HS, Endicott J, Leon AC et al (2000). Does incomplete recovery from first lifetime major depressive episode herald a chronic course of illness? Am J Psychiatr 157: 1501–1504.
Kahan L (2000). The CDRH Staff College, The Least Burdensome Provisions of the FDA Modernization Act of 1997. Presentation delivered on March 19, 2000.
Kauffmann CD, Cheema MA, Miller BE (2004). Slow right prefrontal transcranial magnetic stimulation as a treatment for medication-resistant depression: a double-blind, placebo-controlled study. Depress Anxiety 19: 59–62.
Kellinghaus C, Loddenkemper T, Moddel G, Tergau F, Luders J, Ludemann P et al (2003). Electric brain stimulation for epilepsy therapy. Nervenarzt 74: 664–676.
Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR et al (2003). The epidemiology of major depressive disorder. Results from the National Comorbidity Survey Replication (NCS-R). JAMA 289: 3095–3105.
Kling MA, Loyd D, Sansbury N, Ren Ke, Murphy AZ (2003). Effects of short-term VNS therapy on Fos expression in rat brain nuclei. Poster presented at the 58th Annual Scientific Convention of the Society of Biological Psychiatry. San Francisco, CA, May 15–17, 2003.
Kovacs DA, Zoll JG (1974). Seizure inhibition by median raphe nucleus stimulation in rat. Brain Res 70: 165–169.
Krahl SE, Clark KB, Smith DC, Browning RA (1998). Locus ceruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia 39: 709–714.
Krahl SE, Senanayake SS, Pekary AE, Sattin A (2004). Vagus nerve stimulation (VNS) is effective in a rat model of antidepressant action. J Psychiatr Res 38: 237–240.
Lenox RH, Frazer A (2002). Mechanism of action of antidepressants and mood stabilizers (chapter 79). In: Davis KL, Charney D, Coyle JT, Nemeroff C (eds). Neuropsychopharmacology: The Fifth Generation of Progress. Lippincott Williams & Wilkins: Philadelphia. pp 1139–1163.
Lisanby SH, Bazil CW, Resor SR, Nobler MS, Finck DA, Sackeim HA (2001). ECT in the treatment of status epilepticus. J ECT 17: 210–215.
Lomarev M, Denslow S, Nahas Z, Chae J-H, George MS, Bohning DE (2002). Vagus nerve stimulation (VNS) synchronized BOLD fMRI suggests that VNS in depressed adults has frequency/dose dependent effects. J Psychiatr Res 36: 219–227.
Malhi GS, Bartlett JR (2000). Depression: a role for neurosurgery? Br J Neurosurg 14: 415–422.
Marangell LB, Rush AJ, George MS, Sackeim HA, Johnson CR, Husain MM et al (2002). Vagus nerve stimulation (VNS) for major depressive episodes: one year outcomes. Biol Psychiatr 51: 280–287.
Matthews K, Eljamel MS (2003). Vagus nerve stimulation and refractory depression. Br J Psychiatr 183: 181–183.
Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S et al (2000). Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatr 48: 830–843.
Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C et al (2005). Deep brain stimulation for treatment-resistant depression. Neuron 45: 651–660.
McNamara JO, Galloway MT, Rigsbee LC, Shin C (1984). Evidence implicating substantia nigra in regulation of kindled seizure threshold. J Neurosci 4: 2410–2417.
Michaud CM, Murray CJL, Bloom BR (2001). Burden of disease—implications for future research. JAMA 285: 535–539.
Montoya A, Weiss AP, Price BH, Cassem EH, Dougherty DD, Nierenberg AA et al (2002). Magnetic resonance imaging-guided stereotactic limbic leukotomy for treatment of intractable psychiatric disease. Neurosurgery 50: 1043–1049.
Morris GL, Mueller WM, The Vagus Nerve Stimulation Study Group (1999). Long-term treatment with vagus nerve stimulation in patients with refractory epilepsy. Neurology 53: 1731–1735.
Mu Q, Bohning DE, Nahas Z, Walker J, Anderson B, Johnson KA et al (2004). Acute vagus nerve stimulation using different pulse widths produces varying brain effects. Biol Psychiatr 55: 816–825.
Nahas Z, Marangell LB, Husain MM, Rush AJ, Sackeim HA, Lisanby SH et al (2005). Two-year outcome of vagus nerve stimulation (VNS) for treatment of major depressive episodes. J Clin Psychiatr 66: 1097–1104.
Naritoku DK, Terry WJ, Helfert RH (1995). Regional induction of fos immunoreactivity in the brain by anticonvulsant stimulation of the vagus nerve. Epilepsy Res 22: 53–62.
Nibuya M, Morinobu S, Duman RS (1995). Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci 15: 7539–7547.
Nobler MS, Oquendo MA, Kegeles LS, Malone KM, Campbell CC, Sackeim HA et al (2001). Decreased regional brain metabolism after ECT. Am J Psychiatr 158: 305–308.
Prudic J, Olfson M, Marcus SC, Fuller RB, Sackeim HA (2004). Effectiveness of electroconvulsive therapy in community settings. Biol Psychiatr 55: 301–312.
Rasmussen KG, Sampson SM, Rummans TA (2002). Electroconvulsive therapy and newer modalities for the treatment of medication-refractory mental illness. Mayo Clin Proc 77: 552–556.
Robinson RG (2003). Poststroke depression: prevalence, diagnosis, treatment, and disease progression. Biol Psychiatr 54: 376–387.
Rosenbaum JF, Heninger G (2000). Vagus nerve stimulation for treatment-resistant depression. Biol Psychiatr 47: 273–275.
Rush AJ, Crismon ML, Toprac MG, Trivedi MH, Rago WV, Shon S et al (1998). Consensus guidelines in the treatment of major depressive disorder. J Clin Psychiatr 59 (Suppl 20): 73–84.
Rush AJ, George MS, Sackeim HA, Marangell LB, Husain MM, Giller C et al (2000). Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol Psychiatr 47: 276–286.
Rush AJ, Marangell LB, Sackeim HA, George MS, Brannan SK, Davis SM et al (2005a). Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatr 58: 347–354.
Rush AJ, Sackeim HA, Marangell LB, George MS, Brannan SK, Davis SM et al (2005b). Effects of 12 months of vagus nerve stimulation in treatment-resistant depression: a naturalistic study. Biol Psychiatr 58: 355–363.
Rush AJ, Trivedi MH (1995). Treating depression to remission. Psychiatr Ann 25: 704–705, 709.
Sackeim HA, Haskett RF, Mulsant BH, Thase ME, Mann JJ, Pettinati HM et al (2001b). Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: a randomized controlled trial. JAMA 285: 1299–1307.
Sackeim HA, Rush AJ, George MS, Marangell LB, Husain MM, Nahas Z et al (2001a). Vagus nerve stimulation (VNS™) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology 25: 713–728.
Sackeim HA, Rush AJ, Marangell LB, George MS, Brannan SK (2004). Long-term antidepressant effects of vagus nerve stimulation (VNS) in treatment-resistant depression. Poster presented at 43rd American College of Neuropsychopharmacology Annual Meeting. San Juan, PR, December 12–16, 2004.
Salinsky MC, Uthman BM, Ristanovic RK, Wernicke JF, Tarver WB (1996). Vagus nerve stimulation for the treatment of medically intractable seizures. Results of a 1-year open-extension trial. Vagus Nerve Stimulation Study Group. Arch Neurol 53: 1176–1180.
Schulze-Rauschenbach SC, Harms U, Schlaepfer TE, Maier W, Falkai P, Wagner M (2005). Distinctive neurocognitive effects of repetitive transcranial magnetic stimulation and electroconvulsive therapy in major depression. Br J Psychiatr 186: 410–416.
Sheikh S, Hagen MC, Adson D, Rittberg B, Abuzzahab FS, Lee JT et al (2003). Effects of VNS therapy on brain metabolism in severe, chronic treatment-resistant depression: one-year outcome. Poster presented at the 58th Annual Scientific Convention of the Society of Biological Psychiatry. San Francisco, CA, May 15–17, 2003.
Siegel J, Murphy GJ (1979). Serotonergic inhibition of amygdala-kindled seizures in cats. Brain Res 174: 337–340.
The Vagus Nerve Stimulation Study Group (1995). A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. Neurology 45: 224–230.
Walker BR, Easton A, Gale K (1999). Regulation of limbic motor seizures by GABA and glutamate transmission in nucleus tractus solitarius. Epilepsia 40: 1051–1057.
Yatham LN (2004). Newer anticonvulsants in the treatment of bipolar disorder. J Clin Psychiatr 65 (Suppl 10): 28–35.
Zobel A, Joe A, Freymann N, Clusmann H, Schramm J, Reinhardt M et al (2005). Changes in regional cerebral blood flow by therapeutic vagus nerve stimulation in depression: an exploratory approach. Psychiatr Res 139: 165–179.
Acknowledgements
We thank Sally Laden for editorial support in developing early drafts of this manuscript. We maintained complete control over the direction and content of the paper. Preparation of this report was supported by an unrestricted educational grant from Cyberonics Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nemeroff, C., Mayberg, H., Krahl, S. et al. VNS Therapy in Treatment-Resistant Depression: Clinical Evidence and Putative Neurobiological Mechanisms. Neuropsychopharmacol 31, 1345–1355 (2006). https://doi.org/10.1038/sj.npp.1301082
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301082
Keywords
This article is cited by
-
Transcutaneous auricular vagus nerve stimulation for the treatment of myoarthropatic symptoms in patients with craniomandibular dysfunction – a protocol for a randomized and controlled pilot trial
Pilot and Feasibility Studies (2024)
-
Stress-induced vagal activity influences anxiety-relevant prefrontal and amygdala neuronal oscillations in male mice
Nature Communications (2024)
-
Accelerated TMS - moving quickly into the future of depression treatment
Neuropsychopharmacology (2024)
-
Dosimetry in cranial photobiomodulation therapy: effect of cranial thickness and bone density
Lasers in Medical Science (2024)
-
Klinische Wirksamkeit der aurikulären Vagusnervstimulation in der Behandlung chronischer und akuter Schmerzen
Der Schmerz (2023)