Abstract
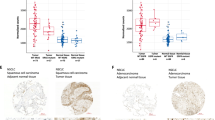
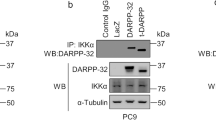
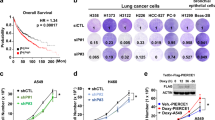
c-Jun N-terminal kinase (JNK) has been reported to either potentiate or inhibit oncogenesis, depending upon the cellular context, but its role in lung neoplasia is unclear. Here we sought to define the role of JNK in lung neoplasia by examining evidence of JNK phosphorylation in non-small-cell lung cancer (NSCLC) biopsy samples and by using genetic and pharmacologic approaches to modulate JNK expression and activity in cultured cells. Immunohistochemical staining for JNK phosphorylation was detected in 114 (45%) of 252 NSCLC biopsy samples and was predominantly nuclear, providing evidence of JNK activation in a subset of NSCLC cases. Introduction of a doxycycline-inducible, constitutively active, mutant mitogen-activated protein kinase kinase 4 (MKK4) into the human bronchial epithelial cell lines BEAS-2B and HB56B increased the cells’ proliferation, migration, invasion and clonogenicity. Depletion of JNK in MKK4 mutant-transformed BEAS-2B cells by introduction of JNK1/2 short hairpin RNA reversed the transformed phenotype, indicating that JNK activation is oncogenic and MKK4 confers neoplastic properties in these cells. The proliferation of NSCLC cell lines HCC827 and H2009, in which JNK and its substrate c-Jun are constitutively phosphorylated, was inhibited by SP600125, a JNK kinase inhibitor. We conclude that JNK is activated in a subset of NSCLC biopsy samples and promotes oncogenesis in the bronchial epithelium, suggesting that strategies to inhibit the JNK pathway should be considered for the prevention and treatment of NSCLC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bachmeier BE, Vené R, Iancu CM, Pfeffer U, Mayer B, Noonan D et al. (2005). Transcriptional control of cell density dependent regulation of matrix metalloproteinase and TIMP expression in breast cancer cell lines. Thromb Haemost 293: 761–769.
Bakiri L, Lallemand D, EWetzel EB, Yaniv M . (2000). Cell cycle-dependent variations in c-Jun and JunB phosphorylation: a role in the control of cyclin D1 expression. EMBO J 19: 2056–2068.
Belguise K, Kersual K, Galtier F, Chalbos D . (2005). FRA-1 expression level regulates proliferation and invasiveness of breast cancer cells. Oncogene 24: 1434–1444.
Bost F, McKay R, Bost M, Potapova O, Dean NM, Mercola D . (1999). The Jun N-terminal kinase 2 isoform is preferentially required for epidermal growth factor-induced transformation of human A549 lung adenocarcinoma cells. Mol Cell Biol 19: 1938–1949.
Briggs J, Chamboredon S, Castellazzi M, Kerry JA, Bos TJ . (2002). Transcriptional upregulation of SPARC, in response to c-Jun overexpression, contributes to increased motility and invasion of MCF7 breast cancer cells. Oncogene 21: 7077–7091.
Cazillis M, Bringuier AF, Delautier D, Buisine M, Bernuau D, Gespach C et al. (2004). Disruption of MKK4 signaling reveals its tumor-suppressor role in embryonic stem cells. Oncogene 23: 4735–4744.
Davies H, Hunter C, Smith R, Stephens P, Greenman C, Bignell G et al. (2005). Somatic mutations of the protein kinase gene family in human lung cancer. Cancer Res 65: 7591–7595.
Gallo TK, Johnson GL . (2002). Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol 3: 663–672.
Geqiang L, Xiang Y, Sabapathy K, Silverman RH . (2004). An apoptotic signaling pathway in the interferon antiviral response mediated by RNase L and cJun N-terminal kinase. J Biol Chem 279: 1123–1131.
Hess P, Pihan G, Sawyers CL, Flavell RA, Davis RJ . (2002). Survival signaling mediated by c-Jun N-terminal kinase in transformed B lymphoblasts. Nat Genet 32: 201–205.17.
Huang C, Jacobson K, Schaller MD . (2004). MAP Kinases and cell migration. J Cell Science 117: 4619–4628.
Huang C, Rajfur Z, Borchers C, Schaller MD, Jacobson K . (2003). JNK phosphorylates paxillin and regulates cell migration. Nature 424: 219–223.
Ip YT, Davis RJ . (1998). Signal transduction by the cJun N-terminal kinase (JNK) -from inflammation to development. Curr Opin Cell Biol 10: 205–219.
Kennedy NJ, Davis RJ . (2003). Role of JNK in tumor development. Cell Cycle 2: 199–201.
Kennedy NJ, Sluss HK, Jones SN, Bar-Sagi D, Flavell RA, Davis RJ . (2003). Suppression of Ras-stimulated transformation by the JNK signal transduction pathway. Genes Dev 17: 629–637.
Kim HL, Vander-Griend DJ, Yang X, Benson DA, Dubauskas Z, Yoshida BA et al. (2001). Mitogen-activated protein kinase kinase 4 metastasis suppressor gene expression is inversely related to histological pattern in advancing human prostatic cancers. Cancer Res 61: 2833–2837.
Kim MH, Yoo HS, Chang HJ, Hong MH, Kim HD, Chung IJ et al. (2005). Cell cycle-dependent variations in c-Jun and JunB phosphorylation: a role in the control of cyclin D1 expression. Biochem Biophys Res Commun 2333: 874–880.
Lee HY, Suh YA, Lee JI, Hassan KA, Mao L, Force T et al. (2002). Inhibition of oncogenic K-ras signaling by aerosolized gene delivery in a mouse model of human lung cancer. Clin Cancer Res 8: 2970–2975.
Lee HY, Suh YA, Xia D, Lu Y, Superty R, LaPushin R et al. (2003). Evidence that phosphatidylinositol 3-kinase- and mitogen-activated protein kinase kinase-4/c-Jun NH2-terminal kinase-dependent pathways cooperate to maintain lung cancer cell survival. J Biol Chem 278: 23630–23638.
Liu Y, Lu C, Shen Q, Medellin DM, Kim H, Brown PH . (2004). AP-1 blockade in breast cancer cells causes cell cycle arrest by suppressing G1 cyclin expression and reducing cyclin-dependent kinase activity. Oncogene 23: 8238–8246.18.
Lorusso PM, Adjei AA, Varterasian M, Gadgeel S, Reid J, Mitchell DY et al. (2005). Phase I and pharmacodynamic study of the oral MEK inhibitor CI-1040 in patients with advanced malignancies. J Clin Oncol 23: 5281–5293.
Lu C, Shen Q, Dupre E, Kim H, Hilsenbeck S, Brown PH . (2005). c-Fos is critical for MCF breast cancer cell growth. Oncogene 24: 6516–6524.
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW et al. (2004). Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350: 2129–2139.
Milde-langosch K, Roder H, Andritzky B, Aslan B, Hemminger G, Brinkmann A et al. (2004). The role of the AP-1 transcription factors c-Fos, FosB, Fra-1 and Fra-2 in the invasion process of mammary carcinomas. Breast Cancer Res Treat 86: 139–152.
Mitsudomi T, Viallet J, Mulshine JL, Linnoila I, Minna JD, Gazdar AF . (1991). Mutations of ras gene distinguish a subset of non-small-cell-lung cancer cell lines from small cell lung cancer cell lines. Oncogene 6: 1352–1362.
Nishina H, Fischer KD, Radvanyi L, Shahinian A, Hakem R, Rubie EA et al. (1997). Stress-signalling kinase Sek1 protects thymocytes from apoptosis mediated by CD95 and CD3. Nature 385: 350–353.
Nishina H, Vaz C, Billia P, Nghiem M, Sasaki T, De la Pompa JL et al. (1999). Defective liver formation and liver cell apoptosis in mice lacking the stress signaling kinase SEK1/MKK4. Development 126: 505–516.
Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S et al. (2004). EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304: 1497–1500.
Parsons DW, Wang TL, Samuels Y, Bardelli A, Cummins JM, DeLong L et al. (2005). Colorectal cancer: mutations in a signalling pathway. Nature 436: 792.
Pollack JR, Perou CM, Alizadeh AA, Eisen MB, Pergamenschikov A, Williams CF et al. (1999). Genome wide analysis of DNA copy number changes using cDNA microarrays. Nat Genet 23: 41–46.
Pollack JR, Sorlie T, Perou CM, Rees CA, Jeffrey SS, Lonning PE et al. (2002). Microarray analysis reveals a major direct role of DNA copy number alteration in the transcriptional program of human breast tumors. Proc Natl Acad Sci USA 99: 12963–12968.
Potapova O, Gorospe M, Bost F, Dean NM, Gaarde WA, Mercola D et al. (2000). c-Jun N-terminal kinase is essential for growth of human T98G glioblastoma cells. J Biol Chem 275: 24767–24775.
Potapova O, Haghigi A, Bost F, Liu C, Birrer MJ, Gjerset R et al. (1997). The Jun kinase/stress-activated protein kinase pathway functions to regulate DNA repair and inhibition of the pathway sensitizes tumor cells to cis-platin. J Biol Chem 272: 14041–14047.
Rangaswami H, Bulbule A, Kundu GC . (2005). JNK1 differentially regulates osteopontin-induced nuclear factor inducing kinase/MEKK1-dependent activating protein-1-mediated promatrix metalloproteinase-9 activation. J Biol Chem 280: 19381–19392.
Reddel RR, Hsu IC, Mass MJ, Hukku B, Gerwin BI, Salghetti SE et al. (1991). A human bronchial epithelial cell strain with unusual in vitro potential, which undergoes neoplastic transformation after SV40T antigen gene transfection. Int J Cancer 48: 764–773.
Reddel RR, Ke Y, Gerwin BI, McMenamin MG, Lechner JF, Su RT et al. (1988). Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate co-precipitation with a plasmid containing SV40 early region genes. Cancer Res 48: 1904–1909.
Su GH, Hilgers W, Shekher MC, Tang DJ, Yeo CJ, Hurban RH et al. (1998). Alterations in pancreatic, biliary, and breast carcinomas support MKK4 as a genetically targeted tumor suppressor gene. Cancer Res 58: 2339–2342.
Sugio K, Kishimoto Y, Virmani AK, Hung JY, Gazdar AF . (1992). Kras gene mutations as a prognostic marker in adenocarcinoma of the human lung without lymph node metastasis. Cancer Res 52: 339–346.20.
Teng DH, Perry III WL, Hogan JK, Baumgard M, Bell R, Berry S et al. (1997). Human mitogen-activated protein kinase kinase 4 as a candidate tumor suppressor. Cancer Res 57: 4177–4182.
Vicent S, Garayoa M, Lopez-Picazo JM, Lozano MD, Toledo G, Thunnisen FBJM et al. (2004). Mitogen-activated protein kinase phosphatase-1 is overexpressed in non-small cell lung cancer and is an independent predictor of outcome in patients. Clin Cancer Res 10: 3639–3649.
Wang L, Pan Y, Dai JL . (2004). Evidence of MKK4 pro-oncogenic activity in breast and pancreatic tumors. Oncogene 23: 5978–5985.
Xiao L, Lang W . (2000). A dominant role for the Jun N-terminal kinase in oncogenic ras-induced morphologic transformation of human lung carcinoma cells. Cancer Res 60: 400–408.
Yamada SD, Hickson JA, Hrobowski Y, Vander Griend DJ, Benson D, Montag A et al. (2002). Mitogen-activated protein kinase kinase 4 (MKK4) acts as a metastasis suppressor gene in human ovarian carcinoma. Cancer Res 62: 6717–6723.
Yang YM, Bost F, Charbono W, Yang YM, Bost F, Charbono W et al. (2003). C-Jun NH(2)-terminal kinase mediates proliferation and tumor growth of human prostate carcinoma. Clin Cancer Res 9: 391–401.
Zhong CY, Zhou YM, Douglas GC, Witschi H, Pinkerton KE . (2005). MAPK/AP-1 signal pathway in tobacco smoke-induced cell proliferation and squamous metaplasia in the lungs of rats. Carcinogenesis 26: 2187–2195.
Acknowledgements
This work was supported by National Institutes of Health Grants R01 CA105155 and P50 CA70907.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Rights and permissions
About this article
Cite this article
Khatlani, T., Wislez, M., Sun, M. et al. c-Jun N-terminal kinase is activated in non-small-cell lung cancer and promotes neoplastic transformation in human bronchial epithelial cells. Oncogene 26, 2658–2666 (2007). https://doi.org/10.1038/sj.onc.1210050
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210050
Keywords
This article is cited by
-
Metastasis suppressors: functional pathways
Laboratory Investigation (2018)
-
Annexin A2 contributes to cisplatin resistance by activation of JNK-p53 pathway in non-small cell lung cancer cells
Journal of Experimental & Clinical Cancer Research (2017)
-
JNKs function as CDK4-activating kinases by phosphorylating CDK4 and p21
Oncogene (2017)
-
Dual modulation of JNK and Akt signaling pathways by chaetoglobosin K in human lung carcinoma and ras-transformed epithelial cells
Investigational New Drugs (2013)
-
The role of the c-Jun N-terminal kinase 2-α-isoform in non-small cell lung carcinoma tumorigenesis
Oncogene (2011)