Abstract
A Ca2+ signaling model is proposed to consider the crosstalk of Ca2+ ions between endoplasmic reticulum (ER) and mitochondria within microdomains around inositol 1, 4, 5-trisphosphate receptors (IP3R) and the mitochondrial Ca2+ uniporter (MCU). Our model predicts that there is a critical IP3R-MCU distance at which 50% of the ER-released Ca2+ is taken up by mitochondria and that mitochondria modulate Ca2+ signals differently when outside of this critical distance. This study highlights the importance of the IP3R-MCU distance on Ca2+ signaling dynamics. The model predicts that when MCU are too closely associated with IP3Rs, the enhanced mitochondrial Ca2+ uptake will produce an increase of cytosolic Ca2+ spike amplitude. Notably, the model demonstrates the existence of an optimal IP3R-MCU distance (30–85 nm) for effective Ca2+ transfer and the successful generation of Ca2+ signals in healthy cells. We suggest that the space between the inner and outer mitochondria membranes provides a defense mechanism against occurrences of high [Ca2+]Cyt. Our results also hint at a possible pathological mechanism in which abnormally high [Ca2+]Cyt arises when the IP3R-MCU distance is in excess of the optimal range.
Similar content being viewed by others
Introduction
The calcium ion (Ca2+) is a ubiquitous intracellular signal controlling diverse cellular functions, such as muscle cell contraction, neurotransmitter release from neurons and astrocytes, metabolic processes, egg activation and cell maturation, differentiation and death1. Ca2+ signals commonly appear as repetitive spikes of cytosolic Ca2+ concentration ([Ca2+]Cyt), with signaling information encoded in the frequency, amplitude and duration of these oscillations2. The complex spatial-temporal profiles of [Ca2+]Cyt depend heavily on the endoplasmic reticulum (ER) and mitochondria3 which act as the two major intracellular Ca2+ stores.
While it has been widely accepted that the inositol 1, 4, 5-trisphosphate receptors (IP3R) function as Ca2+ release channels on the ER membrane1, mitochondria were previously considered only to function as Ca2+-sinks under pathological conditions when there is an abnormally high cellular Ca2+1. This view on mitochondria began to change in the 1990s with two landmark studies demonstrating that non-pathological elevations of [Ca2+]Cyt are accompanied by a marked rise of mitochondrial matrix Ca2+ concentrations ([Ca2+]Mt)4 and that [Ca2+]Mt responds dynamically to physiological oscillations of [Ca2+]Cyt5. Experimental observations indicated that mitochondria can accumulate Ca2+ through the mitochondrial Ca2+ uniporter (MCU) which is located on the inner mitochondrial membrane (IMM)6. In despite of its low Ca2+ affinity, the MCU is exposed to a high [Ca2+]Cyt generated in microdomain around the channel pore of open IP3Rs7,8, thus even when global [Ca2+]Cyt is low a high value of [Ca2+]Mt may be observed. As a result, mitochondria can serve as buffers of cytoplasmic Ca2+ under both pathological and non-pathological conditions.
Although it is now generally believed that mitochondria function as a dynamic Ca2+ sequestration system to shape cytosolic Ca2+ oscillations and therefore regulate many physiological processes6, the detailed mechanism underlying how mitochondria influence global Ca2+ signals remains elusive. Contradictory mechanisms have been suggested on how mitochondrial Ca2+ uptake regulates IP3R activity, including up-regulation9,10,11, down-regulation12,13 and complete independence14. Furthermore, although mitochondrial Ca2+ uptake via MCU depends primarily on the spacing between the ER and mitochondria15, the working distance between the two organelles remains unclear due to the complexity of the intracellular environment and the lack of spatial resolution in the experimental observations. Indeed, the existing estimates of the distances between the ER and mitochondria vary hugely, from less than 10 nm to more than 200 nm16. A recent paper published in PNAS17 even argued that the mitochondria may not act as a significant dynamic buffer of cytosolic Ca2+ under physiological conditions.
Computational models provide a valuable tool for understanding of the mechanisms underlying Ca2+ signal transduction18 and the dynamics of Ca2+ release from the ER has been extensively modeled19,20,21,22,23. Some models only focused on mitochondrial Ca2+ dynamics24,25. Dash and co-workers developed a detailed kinetic model for MCU to match published data sets on mitochondrial Ca2+ uptake25. However, the involvement of both the ER and mitochondria in Ca2+ dynamics has only been considered in a limited number of models18,26,27,28,29,30. In the earlier models18,26, it was postulated that the MCU substantially sequesters Ca2+ when [Ca2+]Cyt < 1 μM, in contrast to experimental evidence that the MCU actually sequesters Ca2+ in the range of 10–20 μM3,31. Dupont et al.28 modeled the effect of Hint2, a mitochondrial protein, on cytosolic Ca2+ dynamics in hepatocytes. However, in these models the maximum value of ER luminal [Ca2+] ([Ca2+]ER) is in the order of several to several tens of μM, deviating drastically from most of the measured figures of 100–900 μM32 and the value of [Ca2+]Mt was also significantly underestimated. More importantly, except the model proposed by Szopa et al.29, none of them take into account the high [Ca2+]Cyt microdomain, which is critical for mitochondrial Ca2+ signaling. The model considered by Szopa et al.29 is a modification of the model from ref. 26, analyzing the influence of microdomain on the period and shape of calcium oscillations. They supposed that MCU sense elevated Ca2+ concentration which is directly equal to that in ER29. This assumption is incoherent with the experimental observation that [Ca2+]Cyt in microdomain is about several tens of μM, which is about 10-fold higher than that in the bulk cytosol7 and about 10-fold lower than that in ER32. Thus, there is a lack of model to quantitatively investigate how the crosstalk between the ER and mitochondria controls Ca2+ signaling.
Here we consider a more realistic model to investigate the role of mitochondria in Ca2+ signaling to couple the ER and mitochondria based on the Ca2+ microdomain. A cytosolic microdomain is specifically considered in order to discuss the MCU dynamics and so each MCU specifically responds to the local high Ca2+ concentration in microdomain generated by a cluster of IP3Rs. Our results demonstrate the critical role of mitochondrial Ca2+ uptake in modulating IP3R-released Ca2+ signaling. We show that the location of the MCU relative to IP3Rs is a key determinant for modulating Ca2+ signals. There is a critical IP3R-MCU distance at which 50% of the ER-released Ca2+ is taken up by mitochondria and that mitochondria modulate Ca2+ signals differently when outside of this critical distance. When the IP3R-MCU distance is greater than the critical distance mitochondrial Ca2+ uptake stimulates ER-Ca2+ release by rapidly reducing the amplitude of the [Ca2+]Cyt signal within the microdomain and suppressing the inhibition dynamics of high [Ca2+]Cyt on IP3R. However, when the distance is less than the critical distance, mitochondrial Ca2+ uptake demonstrates a different effect on IP3R dynamics. Initially strong mitochondrial uptake forces [Ca2+]Cyt to a low level, thus preventing the strong activation dynamics of [Ca2+]Cyt on IP3R. This leads to strong IP3R inhibition on the decay of subsequent [Ca2+]Cyt spikes. Thus, our model predicts that when MCU are too closely associated with IP3Rs, the enhanced mitochondrial Ca2+ uptake will produce an increase of cytosolic Ca2+ spike amplitude. As a result, there is an optimal spacing about 30–85 nm between the ER and mitochondria for generation of experimentally observed Ca2+ oscillations in living cells.
Modelling coupled ER and mitochondria Ca2+ dynamics
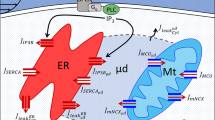
A “closed” cell model, which does not include Ca2+ exchange between cytosol and external medium through the plasma membrane, is considered to investigate the coupling effects between the ER and mitochondria on Ca2+ signaling (Fig. 1(a)). As a result, the model has three compartments: cytosol (Cyt), ER and mitochondria (Mt). As shown in Fig. 1(a), the dynamics of the free [Ca2+] in these compartments is determined by the Ca2+ fluxes from various channels and pumps and by buffering processes with various Ca2+ binding proteins (BP), given by,



The model design.
(a) The schematic diagram of the components and fluxes included in the model. When a cell is stimulated to generate IP3, IP3 binds to IP3R and triggers Ca2+ release from the ER through IP3Rs. The Ca2+ in the cytsol can be pumped back into ER by SERCA. (b) IP3Rs and MCUs in close proximity form a microdomain, a half 3-dimensional cytosolic space separated by ER membrane. A high microdomain [Ca2+] ([Ca2+]Mic) can be generated upon opening of IP3Rs which in turn leads to Ca2+ uptake into mitochondria through MCUs. Ca2+ is extruded from mitochondria back into the cytosol by NCX. (c) The four-state model of MCU monomer with binding and unbinding rates.
Here [Ca2+] represents the free [Ca2+], V the volume of three compartments, jin the Ca2+ flux from outside to inside and jout the opposite flux. The three terms jCa represent Ca2+ dissociated from the BP.
In each compartment, Ca2+ can be buffered by various BPs. The kinetics between free Ca2+ and BP is described by a simple chemical reaction

giving

where [BP]Tot_i and [CaBP]i represent the total concentration of BP and the concentration of Ca2+-bound BP in each compartment, kon and koff denote the on and off rate constants of Ca2+ with the BP, respectively.
Ca2+ fluxes in ER component
The Ca2+ effluxes from the ER to cytosol are given by the IP3R release and a passive leakage with the concentration gradient across the ER membrane as their driving force, i.e.,

with vIP3R being the maximal efflux from the IP3Rs in the ER membrane, PoIP3R the open fraction of IP3Rs and cLeak the Ca2+ leak constant.
The IP3R channel is an assembly of four equivalent subunits, each of which is mutually gated by IP3 and Ca2+. The gating dynamics of each IP3R subunit is described by the Li-Rinzel model19 and we assumed that the channel is open if at least three of its subunits are in the active state20. Thus, we get the following expression for PoIP3R:

Here sact is the probability in the active state of the subunit, [IP3] is the IP3 concentration and the slowly gating variable h is given by

where ai and di are constants.
The cytosolic Ca2+ can be pumped back into ER by active SERCA which is given by,

where vSERCA and kSERCA are maximum SERCA pump flux and SERCA activation constants.
Ca2+ fluxes in mitochondrial component
Mitochondrial Ca2+ uptake through MCU is driven primarily by the large voltage across IMM. Besides the IMM voltage, it has been shown that MCU flux is also regulated by MICU33. For simplicity, in our model we only consider the role of MICU1 which has been well investigated in experiment. Then the MCU influx can be expressed as

Here vMCU is the maximal flux of MCU, ΔΦ is the voltage driving force, RMICU represents the MICU1 regulatory term and PoMCU describes the open fraction of MCU.
The IMM voltage Ψ is 150 ~ 180 mV (negative inside). Although the net Ca2+ flux into the mitochondria might associate with a reduction in voltage, some evidence suggests that mitochondrial Ca2+ uptake does not appear to alter Ψ significantly during physiological condition34. With the assumption of constant Ψ, the driving force for MCU can be given by35

Here F, R and T are the Faraday constant, the gas constant and the Kelvin temperature, respectively; b and Ψ0 are fitting parameters35.
As a regulatory subunit, MICU1 senses [Ca2+] in mitochondrial intermembrane space to set a threshold for MCU33. The influx rate of MCU only becomes substantial when the extra-mitochondrial [Ca2+] reaches values above 3 μM36. As a result, the regulation of MICU1 to MCU activity has a sharp activation threshold (kMICU) with an apparent Hill coefficient equal to 436:

where [Ca2+]Mic is the microdomain [Ca2+]Cyt that MCU is exposed to, which will be discussed later.
Whereas MICU1 acts as a regulator, MCU represents the core pore-forming component responsible for mitochondrial Ca2+ uptake37,38. Recent experimental and bioinformatic analysis reveals that MCU is an oligomer37 and most likely a tetramer39. Considering the biphasic dependence of MCU on [Ca2+]Cyt, i.e., fast Ca2+-dependent activation and slow Ca2+-dependent inactivation of the MCU31, we simply propose that each monomer has two Ca2+ binding sites, one for activation and another for inhibition. Therefore a monomer can exist in 4 states of s00, s10, s01 and s11 with the first index for activation binding site and the second index for inhibition binding site and with 1 for binding and 0 unbinding (Fig. 1(c)). Similar as the IP3R, we posit that the MCU is open if either 3 or 4 of the 4 monomers are in the active state (s10). Thus, the MCU open fraction is given by,

where xij denotes the probability of a monomer in states sij. The dynamical equations governing the state of a monomer are



with x00 + x01 + x10 + x11 = 1.
Ca2+ is extruded from mitochondria primarily by NCX which swaps 3 Na+ ions with 1 Ca2+ ion during each exchange cycle, Thus, the kinetic of outward calcium flux can be described as35

where [Na+]Cyt is Na+ concentration in the cytosol, vNCX is maximal NCX activity, both kNa and kNCX are activation constants for NCX.
Microdomain crosstalk between the ER and mitochondria
Now we describe a microdomain model that represents the crosstalk occurring between closely situated ER and mitochondria (Fig. 1(b)). The interacting players are IP3Rs and MCUs in the microdomain. A microdomain of local high [Ca2+]Cyt produced by nearby open IP3Rs is essential for high capacity Ca2+ transport into mitochondria via MCU. It has been reported that 90% of IP3Rs are located close to mitochondria and the majority of MCU sense the high [Ca2+]Mic in the vicinity of IP3Rs29. Here we make a specific assumption that MCU senses the local high [Ca2+]Mic generated by the opening of clustered IP3Rs, but other Ca2+ handling components, such as IP3Rs, SERCA, NCX, etc., are determined by the global averaged [Ca2+]Cyt. The pros and cons of our method will be discussed in the last section of the paper.
It has been suggested that each mitochondrial Ca2+ uptake site must face multiple IP3Rs for effective mitochondrial Ca2+ uptake40. Recent experiment evidence indicates that IP3Rs are typically distributed in clusters on the ER membrane, with each cluster containing a handful of IP3Rs41,42. Patch clamp experiments also show that the Ca2+ current (IsIP3R) passing through a single open IP3R is linearly correlated with the concentration gradient ([Ca2+]ER - [Ca2+]Cyt) across the ER membrane with a slope of SIP3R = 0.3 pA/mM43. Considering the closely distributed IP3Rs, we treat the Ca2+ flux from clustered IP3Rs as a Ca2+ current from a point source at cluster center. As a result, an effective Ca2+ current from clustered IP3Rs is expressed as

Here, PoIP3R is the IP3R open probability given by Eq. 6 and nIP3R is the IP3R number in a cluster. According to Ref. 41, we consider a typical cluster with nIP3R = 4. These 4 IP3Rs are in tight association, organized in a square pattern.
Around the center of IP3R cluster, the open IP3Rs will generate a very sharp distribution of [Ca2+]Cyt due to rapid Ca2+ diffusion producing a microdomain of high [Ca2+]Cyt. Here we also assume that the ER membrane acts as a flat boundary to limit the Ca2+ diffusion in a half 3-dimensional space, i.e. the cytosolic microdomian (Fig. 1(b)). Using hemispherical symmetry of Ca2+ diffusion from a point release source, the sharp distribution around the release point can be established with a signal equation analogous to the linear cable equation44,45. As a solution of the linearized reaction-diffusion equation, the microdomain [Ca2+] is given by45

with

Here, Dc is the diffusion coefficient for free Ca2+. The parameter λ is an important factor that determines the sharp decay of [Ca2+] within a microdomain, which accounts for the Ca2+ diffusion and binding to BPs. [BP]Mic is the total concentration of BP in the microdomain and Kd is its dissociation constant44,45. One can see that the [Ca2+]Mic within a microdomain becomes the cellular average [Ca2+]Cyt when there are no open IP3Rs.
Eq. 16 describes the [Ca2+] distribution at distance r around the point source, i.e., the IP3R cluster center. In our model the parameter r is the distance between the MCU and the IP3R cluster center, which we will call the IP3R-MCU distance in the paper. Thus, [Ca2+]Mic is the microdomain Ca2+ concentration that the MCU sees. Considering MCUs located on IMM, here we also simply assume that the outer mitochondrial membrane (OMM) has little effect on the Ca2+ diffusion as it has high permeability to Ca2+ 6. If we suppose the IMM and ER membrane are parallel, parameter r also represents the distance between IMM and ER membrane. As a result, Eq. 16 represents the microdomain coupling between the ER and mitochondria in our model.
Parameter values
The parameters used in this model are listed in Table 1 (see supplementary material). They are categorized on the basis of their source to: (a) those extracted directly from experimental data; (b) those obtained from previously published models; or (c) the free parameters which were fit to experimental results. The model presented in this study permits simultaneous calculation of [Ca2+] changes in the 3 compartments. Currently, it is difficult to measure experimentally Ca2+ concentrations in the cytosol and ER or mitochondria simultaneously because of the highly dynamic nature of the Ca2+ signals that occur within organelles46. Only a few existing experimental data sets46,47 consist of measurements from all 3 compartments. The free parameters used in our model were extrapolated from fits to these data sets.
Results
The Ca2+ oscillations
Our first priority was to determine whether the model realistically simulated cellular Ca2+ dynamics. As shown in Fig. 2a, when setting [IP3] = 0.5 μM our model generates baseline Ca2+ oscillations occurring as a series of discrete spikes. The values for basal and stimulated [Ca2+] for the cytosol, mitochondria and ER in the model are in close accord with experimental data38,48. The oscillations period is in the order of several ten seconds, as seen in many experimental observations with non-exitable cells2,48. The Ca2+ spikes show relatively rapid rising phases and slow falling phases, mimicking those seen in experimental recordings49. The cytosolic and mitochondrial [Ca2+] oscillate almost in phase, with [Ca2+]Cyt as the leader, while the ER [Ca2+] is in anti-phase with them, also as observed experimentally13,47.
Mitochondria serve as Ca2+ reservoirs.
(a) Oscillations of: [Ca2+]Mic in the IP3R-MCU microdomain, [Ca2+]Cyt in the cytosol, [Ca2+]Mt in mitochondria and [Ca2+]ER in the ER in the presence of mitochondria with a IP3R-MCU distance of 35 nm. The width at half maximum, the rising duration and decay duration of [Ca2+]Cyt spike are marked in the second panel of (a). (b) Oscillations of: [Ca2+]Cyt in the cytosol and [Ca2+]ER in the ER in the absence of mitochondria. [IP3] = 0.5 μM. The unit of [Ca2+] is μM.
Having established that the salient features of the Ca2+ dynamics of our model are in good agreement with existing experimental observations, we examined the effect of manipulating the mechanisms modulated by the crosstalk of the ER and mitochondria. Thus, we also performed simulations in which mitochondria were absent from the model (Fig. 2b). The minimal values of [Ca2+]ER are 311 μM and 276 μM in the absence and in the presence of mitochondria, respectively (Fig. 2), indicating that more Ca2+ ions are released from the ER during each spiking cycle in the presence of mitochondria. The maximal values of [Ca2+]Cyt are 5.6 μM and 2.5 μM in the absence and in the presence of mitochondria, respectively (Fig. 2), showing that mitochondria can significantly decrease [Ca2+]Cyt oscillation amplitude.
Ca2+ oscillation modulated by IP3R-MCU distance
Next we looked at the effect of IP3R-MCU distance on Ca2+ oscillation in detail. Fig. 3 compares oscillating [Ca2+] trajectories in microdomain, ER, mitochondria and cytosol during a single cycle at varying IP3R-MCU distances. By decreasing the IP3R-MCU distance from infinity (we used simulations in which mitochondria were absent from the model as the infinite IP3R-MCU distance), the [Ca2+]Mic in microdomain increases according to Eq. 16 against distance (Fig. 3a), giving an increase in mitochondrial Ca2+ uptake. As a result, the minimal [Ca2+]ER steadily decreases (Fig. 3b) and the [Ca2+]Mt spike amplitude steadily increases until saturating at about 65 μM (Fig. 3c). However, an unexpected finding is that the [Ca2+]Cyt spike amplitude first decreases but then increases as the distance decreases (Fig. 3d).
The time evolution of [Ca2+]Mic (a), [Ca2+]ER (b), [Ca2+]Mt (c) and [Ca2+]Cyt (d) in an oscillating cycle at distance r = 200 (green), 100 (purple), 40 (red), 20 (dark) and 10 nm (grey). The brown lines in (b) and (d) are in the absence of mitochondria. In (a) and (b) the duration of an abrupt increase in Ca2+ release is marked by arrows for r = 10 nm. [IP3] = 0.5 μM.
The critical IP3R-MCU distance
The amplitudes of [Ca2+]Mic, [Ca2+]Mt and [Ca2+]Cyt are plotted in detail as a function of distance from 10 to 150 nm at [IP3] = 0.5 μM in Fig. 4a and 4b. A sharp increase is observed for [Ca2+]Mic and [Ca2+]Cyt at r < 30 nm and [Ca2+]Cyt reaches its minimum at r = 30 nm. Fig. 4b also shows that the [Ca2+]Cyt spiking period increases monotonically from 30 to 50 sec, following the increased ability of mitochondria to take up Ca2+ as the IP3R-MCU distance diminishes. The [Ca2+]Cyt spiking amplitude is determined by the number of Ca2+ ions that diffuse into the cytosol, which is related to the total amount of Ca2+ ions released from ER through IP3Rs and the fraction which are taken by mitochondria. In the model, the total amount of Ca2+ released from the ER through IP3R and the fraction accumulated by mitochondria through MCU during each oscillating cycle are plotted in Fig. 4c as a function of IP3R-MCU distance. The fraction of mitochondrial Ca2+ uptake increases monotonically with decreasing distance indicating an increase in the ability of the mitochondria to take up Ca2+ at closer distances. Our simulation shows that at 30 nm distance, about 50% of the ER-released Ca2+ ions are taken up by mitochondria (see green line with stars in Fig. 4c). As a consequence, rcrt = 30 nm acts as a critical distance for mitochondria to modulate cytosolic Ca2+ signaling: At r > rcrt, more ER-released Ca2+ ions will diffuse into the cytosol than be taken up by mitochondria; whilst at r < rcrt, mitochondria will capture the majority of Ca2+ ions released from ER.
Ca2+ dynamics modulated by the IP3R-MCU distance.
(a) The spike amplitudes of [Ca2+]Mic (triangles) and [Ca2+]Mt (circles). (b) The spike amplitudes of [Ca2+]Cyt (diamonds) and spike period (spheres). (c) The total amount of Ca2+ released from the ER through IP3Rs during each cycle (squares), the amount of Ca2+ released from the ER through IP3Rs during the rising time (circles) and the fraction delivered to mitochondria through MCU (stars). (d) The spike width (triangles), the rising time (squares) and the decay time (circles) of [Ca2+]Cyt oscillations. [IP3] = 0.5 μM.
Ca2+ dynamics modulated by IP3R-MCU distance
Surprisingly, Fig. 4c also shows another unexpected finding that the total amount of Ca2+ released from the ER first increases with distance down to r = 20 nm and then decreases at smaller distance (see black line with squares in Fig. 4c). This raises a critical question: with decreasing distance at r < 20 nm, how can [Ca2+]Cyt spiking amplitudes increase (violet line with diamonds in Fig. 4b), while the total amount of Ca2+ released from the ER decreases (black line with squares in Fig. 4c) but mitochondrial Ca2+ uptake fraction increases (green line with stars in Fig. 4c)?
In order to further examine this conflicting effect of distance on [Ca2+]Cyt spike amplitude and the amount of ER-released Ca2+, we investigated how mitochondrial Ca2+ uptake modulates the spike width, the spike rising time and decay time of [Ca2+]Cyt at varying IP3R-MCU distance. Here the spike width is defined as the duration at half maximum of [Ca2+]Cyt spike amplitude, the rising time is defined as the duration from twice of the minimal [Ca2+]Cyt to the peak of the response and the decay time as the duration from the peak of the response to twice of the minimal [Ca2+]Cyt. An example of definition of these parameters is marked in Fig. 2a for spike curve at distance r = 35 nm.
We first discuss the simple situation at r > rcrt. As shown in Fig. 4d when r > rcrt, the spike width increases with decreasing distance, which primarily arises from an increase in the duration of the decay time, whereas the rising time is rapid and shows little change with distance. As r decreases, the ability of mitochondrial Ca2+ uptake increases, leading to a decrease in [Ca2+]Cyt amplitude. The decrease in [Ca2+]Cyt amplitude in turn leads to a weakening of the Ca2+ inhibition of IP3R dynamics, resulting in the prolonged spike decay time with decreasing distance.
Differently, at r < rcrt, as the IP3R-MCU distance decreases, even though the rising time increases (black line in Fig. 4d), the spike width actually becomes smaller due to a decrease in the decay time (blue and red lines in Fig. 4d). At this small distance, the rising time consists of both a slow rising phase and an abrupt rising phase. As an example, the slow rising and abrupt rising phases for a spike at distance r = 10 nm are indicated by arrows in Fig. 3a&b. The mitochondrial Ca2+ uptake is so strong that, at the beginning of each oscillating cycle, most of the ER-released Ca2+ ions are directly driven into mitochondria, leading to a rapid increase of [Ca2+]Mt (Fig. 3c), but a very slow increase of [Ca2+]Cyt (Fig. 3d). With low [Ca2+]Cyt, there are a small fraction of activating IP3Rs which remain open for a long time, resulting in a slow rise phase for [Ca2+]Cyt (e.g. the gray dash line for r = 10 nm in Fig. 3d with time from 0 to 30 sec). Such a long duration with low [Ca2+]Cyt actually contributes little to the spike width. When [Ca2+]Mt increases to approach to its saturation state, most of the ER-released Ca2+ ions will then diffuse into cytosol. Because of the Ca2+ induced Ca2+ release, there occurs an abrupt increase in the numbers of open IP3Rs, generating a spike (e.g. the gray dash line for r = 10 nm in Fig. 3d with time from 30 to 35 sec).
With shorter IP3R-MCU distance, the mitochondria become more saturated prior to the abrupt rising phase and thus during the abrupt rising phase the Ca2+ ions released from the ER diffuse more into cytosol, generating a higher amplitude spike in [Ca2+]Cyt (violet line with diamonds in Fig. 4b at r < 30 nm). In turn, the elevated [Ca2+]Cyt peak induces a strong inhibitory effect on IP3R dynamics, such that spikes decay faster as the IP3R-MCU distance decreases (red line with circles in Fig. 4d).
The changes with distance seen in the amount of Ca2+ ions released from the ER during the rise and decay phases can then explain why the total amount of ER-released Ca2+ reaches a maximum around r = 20 nm. At r > rcrt, as the rising time is fast and little affected by distance (close to 6 s as shown in Fig. 4d), the elevation in the total amount of ER-released Ca2+ with decreasing distance mainly related to the increase of the decay phase. By decreasing r at rcrt > r > 20 nm, although the amount of ER-released Ca2+ ions during the decay phase decreases because of the shortened decay duration (red line in Fig. 4d), the amount of ER-released Ca2+ ions during the rising phase increases because of the prolonged rising duration (black line in Fig. 4d). As a result, the total amount of Ca2+ released by ER keeps increasing with decreasing r for rcrt > r > 20 nm. However, at very short distance of r < 20 nm, during the rising phase the ER-released Ca2+ ions almost saturate both mitochondria and the cytosol, giving a maximal Ca2+ amount released by ER during the rising phase (see the open circles in Fig. 4c at r < 20 nm). Then, the change of the total amount of ER-released Ca2+ is typically determined by the change of decay time, leading to a decrease in the total amount of ER-released Ca2+ with decreasing distance at r < 20 nm.
Thus we can see how the apparently paradoxical relationship between [Ca2+]Cyt spike amplitude and total amount of ER-released Ca2+ at IP3R-MCU distances <20 nm develops. At r < 20 nm, the [Ca2+]Cyt spike amplitude is mainly determined by the amount of Ca2+ released during the abrupt rising phase of a Ca2+ response, giving an increasing relationship with decreasing distance. In contrast to this, the change in the total amount of ER-released Ca2+ mainly follows the behavior of the spike decay time, giving a decreasing relationship with decreasing distance at r < 20 nm.
The synchronization of the oscillation phases between [Ca2+]Cyt, [Ca2+]ER and [Ca2+]Mt is also modulated by the IP3R-MCU distance. Our data (not shown here) indicate that at r > rcrt, [Ca2+]Cyt peaks first, followed by [Ca2+]ER minimum and then [Ca2+]Mt peak, due to an increase of the amount of ER-released Ca2+ ions diffusing into the cytosol at greater distances. However, when r < rcrt, more ER-released Ca2+ ions are sequestered into mitochondria, so [Ca2+]Mt peaks first, followed by [Ca2+]ER minimum and then [Ca2+]Cyt peak.
Ca2+ dynamics modulated by [IP3]
In the following discussion, we investigate how [IP3] controlled [Ca2+]Cyt oscillations can be modulated by the IP3R-MCU distance. The bifurcation diagram for [Ca2+]Cyt oscillation (i.e. the steady state of [Ca2+]Cyt or the maximum and minimum of [Ca2+]Cyt oscillation) with [IP3] as the bifurcation parameter is plotted at different IP3R-MCU distances in Fig. 5a. When IP3 levels are low, [Ca2+]Cyt can be seen to remain in a low stable steady state. Beyond a certain threshold value of [IP3], [Ca2+]Cyt undergoes periodical oscillations. We also found that, at distance range of 27–122 nm, the system only demonstrates sustained Ca2+ oscillations when [IP3] values are in an intermediate range, evolving into a stable steady state at higher [IP3] (Fig. 5b). Such a bifurcation diagram for r = 60 nm is depicted in the inset of Fig. 5b. Similar bifurcation behavior has been observed in the experimental Ca2+ waves in Xenopus laevis oocytes. In oocytes it has been shown that repetitive Ca2+ waves occur only at intermediate [IP3] between 0.1 and 1 μM50. Thus, as shown in the inset of Fig. 5b, r = 60 nm is the optimum IP3R-MCU distance in which our model replicates these experimental observations. According to the experiment50, for a biologically meaningful Ca2+ oscillation model there should be a right bifurcation point for high [IP3], beyond which there occurs no Ca2+ oscillation. As plotted in Fig. 5b, only in the range of r of 27–122 nm the model can produce Ca2+ oscillations in an intermediate [IP3] range. Outside of such distance range the Ca2+ oscillations always occur even at infinitely high [IP3] (Fig. 5b).
Ca2+ dynamics modulated by [IP3].
(a) The bifurcation diagram (the stable steady states and the maxima and minima of Ca2+ oscillations) of the model with [IP3] as the bifurcation parameter at distance r = 200 (green), 100 (purple), 40 (red), 20 (dark) and 10 nm (grey). (b) The value of [IP3] at the right bifurcation point plotted against distance. The inset is the bifurcation diagram at distance r = 60 nm, in which the arrow marks the right bifurcation point. (c) The maximal [Ca2+]Cyt against the IP3R-MCU distance for [IP3] = 0.1 (lozenges), 0.5 (squares), 1.0 (circles), 2.0 (triangles) and 5.0 μM (stars). The dashed and dotted lines represent [Ca2+]Cyt = 4 and 5 μM, respectively.
[Ca2+]Cyt oscillations with limited amplitudes regulate a host of vital cell functions1. A global [Ca2+]Cyt less than 3.5 μM is usually observed in living cell38,48. However, high [Ca2+]Cyt will trigger and modulate apoptosis51. Fig. 5c shows the maximal [Ca2+]Cyt values seen in our model plotted against IP3R-MCU distance for different [IP3]. One can see that a rapid elevation in [Ca2+]Cyt amplitude is observed at IP3R-MCU distances around 10 nm. A global [Ca2+]Cyt which is smaller than 4.0 or 5.0 μM (i.e. dashed or dotted line in Fig. 5c) is typically obtained with IP3R-MCU distances of 20 to 85 nm or distances of 15 to 150 nm, respectively.
Robustness with varying clustered IP3R number
In above study, we only consider a cluster in which nIP3R = 4 IP3Rs correspond to a single MCU. Experimental evidence suggests that IP3R clusters typically contain a handful (~4) of IP3Rs41,42. Next we discuss the robustness of the model's behavior upon slight changes in nIP3R (from 4 to 3 and 5). By calculating the fraction of Ca2+ ions accumulated by mitochondria compared to the total amount of Ca2+ released from the ER, we see that the critical distance is changed to 20 nm for nIP3R = 3 and 35 nm for nIP3R = 5 (Fig. 6(a)). Fig. 6(b) gives the value of [IP3] at the right bifurcation point against distance with varying nIP3R. A common region to find the right bifurcation [IP3] is obtained with distances of 33 to 93 nm. Fig. 6(c) shows the maximal [Ca2+]Cyt plotted against IP3R-MCU distances for varying nIP3R. A global [Ca2+]Cyt which is smaller than 4.0 or 5.0 μM (i.e. dashed or dotted line in Fig. 6(c)) is typically obtained with IP3R-MCU distances of 25 to 85 nm or distances of 20 to 110 nm, respectively.
Robustness of Ca2+ dynamics on nIP3R.
(a) The fraction of Ca2+ accumulated by mitochondria of the total amount of Ca2+ released from the ER during each oscillating cycle plotted against IP3R-MCU distance. (b) The value of [IP3] at the right bifurcation point against distance. (c) The maximal [Ca2+]Cyt against the IP3R-MCU distance at saturating [IP3] = 5.0 μM. The dashed and dotted lines represent [Ca2+]Cyt = 4 and 5 μM, respectively. Here nIP3R = 3 (stars), 4 (circles) and 5 (triangles).
Taken together, compared with experimental observations38,48,50,51, the simulation results shown in Fig. 5 and Fig. 6 suggest that the optimal IP3R-MCU distance for physiological Ca2+ signaling should be around 30 to 85 nm. When the IP3R-MCU distance is between 30 and 85 nm, as illustrated in Fig. 6(a) approximately 15–55% of ER-released Ca2+ ions can be taken up by mitochondria, compatible with experimental measurements made in various cells52,53. Our model thus suggests that mitochondria, typically working at a short distance from the ER (but larger than rcrt), serve as Ca2+ sinks, sequestering Ca2+ ions released from the ER.
Discussion
Since the 1990s, the manner in which mitochondrial Ca2+ uptake through MCU shapes intracellular Ca2+ signaling has attracted much attention6. The key questions presently debated are: to what extent mitochondria acquire Ca2+; what impact mitochondria have on cytosolic Ca2+ signals; and what dimensions the ER/mitochondria Ca2+ microdomain might have. To address these questions, we constructed a Ca2+ signaling model to consider the Ca2+ crosstalk within microdomains between IP3Rs and MCU. A 4-states MCU model has been suggested based on the latest experimental results31,37,38,39. The MCU is exposed to the microdomain Ca2+ concentration which is released from the nearby clustered IP3Rs34,36, rather than the bulk cytosolic Ca2+ concentration. As a result, the model replicates the shape, amplitude, period and kinetics of the Ca2+ spikes observed in experimental data, allowing us to examine the effects of mitochondrial Ca2+ uptake on cytosolic Ca2+ signals.
From experimental observations, it has been suggested that mitochondrial Ca2+ uptake contributes significantly to the physiological regulation of cytosolic Ca2+38. Regarding the recent controversy whether mitochondria act as significant buffers to cytosolic Ca2+ under physiological conditions17, our model indicates that mitochondria can accumulate large quantities of Ca2+ under physiological conditions (about 15–55% of the total released from the ER) at IP3R-MCU distances of 30–85 nm. Furthermore, our model indicates that the distance between the ER and mitochondria appears to be an important factor in modulating Ca2+ signaling, especially considering that mitochondria can be highly mobile organelles54. There is a critical IP3R-MCU distance rcrt (around 20 to 35 nm depending on nIP3R) at which half of the Ca2+ released by the ER is taken up by mitochondria. At distances smaller than rcrt mitochondria can take up more Ca2+, while at distances larger than rcrt, more Ca2+ ions diffuse from the ER into the cytosol. This leads to mitochondria displaying different kinds of Ca2+ signal modulation depending on their distance from the ER. The effects of mitochondria on the spike amplitude, spike width and rising and decay time of [Ca2+]Cyt spikes, the synchronization phase among [Ca2+]Cyt, [Ca2+]ER and [Ca2+]Mt and the total amount of Ca2+ released from the ER are all substantially modulated by how the IP3R-MCU distance deviates from this critical value.
To explore experimentally the consequences of mitochondrial Ca2+ uptake on global Ca2+ signals, mitochondrial Ca2+ uptake is often prevented directly by applying an MCU inhibitor14 or modulated by using agents which either depolarize or hyperpolarize the mitochondrial membrane potential and thus dissipate or increase the driving force for Ca2+ uptake into the organelle10,14. How mitochondrial Ca2+ uptake affects IP3R release mechanism is still under investigation. Some researchers have argued that mitochondrial Ca2+ uptake may provide a positive modulation of Ca2+ release by suppressing the negative feedback of high [Ca2+]Cyt on IP3R9,10,11. Others have suggested that mitochondrial Ca2+ uptake exerts a negative control on [Ca2+]Cyt by preventing the positive feedback of Ca2+ on IP3R12,13.
In our simulations, the ability of mitochondria to take up Ca2+ is changed by varying the IP3R-MCU distance and so the mitochondrial modulation on IP3R dynamics has been examined. Our model indicates that altering mitochondrial Ca2+ uptake induces a nontrivial change on [Ca2+]Cyt amplitude, which can reconcile the conflicting results of the change of [Ca2+]Cyt amplitude observed in various experiments9,10,11,12,13. When IP3R-MCU distance is larger than the critical distance rcrt, mitochondrial Ca2+ uptake is weak and more Ca2+ ions diffuse into cytosol, the enhancement of mitochondrial Ca2+ uptake causes a decreasing [Ca2+]Cyt spike peak, exerting a weakened inhibition effect on IP3R dynamics, resulting in a prolonged decay time of spikes. Thus, at this region, mitochondrial Ca2+ uptake offers a positive modulation of Ca2+ release by reducing [Ca2+]Cyt amplitude to suppress the inhibition dynamics (i.e. the negative feedback) of high [Ca2+]Cyt on IP3R during decay time of spikes (Fig. 7), similar as suggested in refs 9, 10, 11. While, when IP3R-MCU distance is smaller than the critical distance, mitochondria take more Ca2+ ions than those diffusing into cytosol, the strong mitochondrial Ca2+ uptake will force [Ca2+]Cyt at a small value to prevent the strong activation dynamics (i.e. the positive feedback) of [Ca2+]Cyt on IP3R (Fig. 7), similar as proposed in refs 12, 13. Thus, our model shows that this weak activation effect causes a small Ca2+ release from ER, giving a slow rising phase. When mitochondria approach to saturation and [Ca2+]Cyt increases beyond the threshold of excitable dynamics, an abrupt rising phase occurs to generate a [Ca2+]Cyt spike, giving a high [Ca2+]Cyt amplitude and then inducing a strong inhibitory effect on IP3R dynamics to shorten the decay time of spikes.
Schematic representation of how mitochondria modulate [Ca2+]Cyt.
IP3R channel is regulated by Ca2+ in a biphasic manner: a lower [Ca2+]Cyt promotes Ca2+ release and causes its elevation whereas higher [Ca2+]Cyt inhibits Ca2+ release. There is a critical distance at which 50% of the IP3R-released Ca2+ ions are taken up by mitochondria. When r<rcrt, mitochondrial Ca2+ uptake exerts a negative control on IP3R-released Ca2+ by preventing the positive effect of low [Ca2+]Cyt on IP3R; whilst when r > rcrt, mitochondrial Ca2+ uptake provides a positive modulation on IP3R-released Ca2+ by suppressing the negative effect of high [Ca2+]Cyt on IP3R. Ca2+ oscillations merely occur for moderate values of [IP3] (orange range) with physiological amplitude (gray range), so the optimal distance of IP3R-MCU should be within the intersection zone between the gray and orange range.
An unexpected prediction with the model is that when the IP3R-MCU distance is less than the critical distance, even though mitochondrial Ca2+ uptake keeps increasing with decreasing distance, the total amount of Ca2+ released from the ER decreases while the cytosolic Ca2+ spike amplitude increases. The [Ca2+]Cyt peak is mainly determined by the amount of Ca2+ released during the abrupt rising phase of spikes. At shorter IP3R-MCU distances, the mitochondria become almost saturated before the abrupt rising phase and thus during the abrupt rising phase more of the Ca2+ ions released from the ER diffuse more into cytosol, generating a higher amplitude of [Ca2+]Cyt. However, the change in the total amount of Ca2+ release from the ER seen with decreasing distance is mainly determined by the release of Ca2+ occurring during the decay phase of spike. The enhanced inhibitory effect of higher amplitude [Ca2+]Cyt spikes on IP3R dynamics in turn leads to a shortening of the decay time of the spikes, resulting in a decrease in the total Ca2+ amount released from ER with decreased IP3R-MCU distance.
The existence of high [Ca2+]Mic in microdomains has been put forward as one way to resolve the apparent paradox that even though the Ca2+ affinity of the MCU is low (Kd of 10–20 μM) highly efficient mitochondrial Ca2+ uptake still occurs under physiological conditions with low micromolar global [Ca2+]Cyt3. However, an intriguing question related to the role of the microdomain is the distance between the ER and mitochondria that would be required to control distinct cellular processes. The distance from the ER membrane to OMM was originally estimated to be in excess of 100 nm16, recently it has been suggested that this distance could be in the order of 30 nm15,55. A key prediction of our model is that the optimal distance between the IP3R and MCU is about 30–85 nm. This arises when parameters for [IP3] and [Ca2+] are derived from the experimental observations that Ca2+ oscillations generally occur for intermediate values of IP350 and that physiological [Ca2+]Cyt amplitudes seldom exceed 3.5 μM in living cells38,48, which are illustrated in Fig. 7. Considering that the thickness between OMM and IMM is around 20 nm56, our model predicts that the optimal gap between the ER membrane and OMM should be approximately 10–65 nm, which is in good agreement with measurements obtained via electron microscopy and tomography15,31,55.
Interestingly, our simulation also provides a reason for why MCUs need to be located ~20 nm away from the OMM. In experiment, elevations in the magnitude of [Ca2+]Cyt during typical agonist induced responses could lead to a triggering of [Ca2+]Cyt-induced apoptosis51. Maintaining a distance between IMM and OMM of 20 nm may present a defense mechanism to keep the global [Ca2+]Cyt at a small physiological range. Our model highlights the importance of the IP3R-MCU distance and suggests that potential pathological mechanisms for generating elevated [Ca2+]Cyt and leading to possibly [Ca2+]Cyt-induced apoptosis6,51 would be found in disorders in which the optimal distance between the ER and mitochondria is disturbed. Considering that when untethered to the ER, mitochondria can be highly mobile organelles54 and that little is known of the physiological stimuli governing the distance between the ER and mitochondria, or how the tethers between the ER and mitochondria function or are disrupted during pathology, future research elucidating how the IP3R-MCU distance is maintained and controlled is essential.
Finally, we discuss the advantages and limitations of our model. First, the previous published models typically simulated MCU either by a simple Hill equation26,29 or by the Monod-Wyman-Changeux type allosteric regulation24,27,28. In contrast to these models, we introduced a mechanistic kinetic model to discuss in detail the binding and unbinding processes of Ca2+ to MCU. Second, the numerical simulation about the peak values of [Ca2+]ER or/and [Ca2+]Mt were usually underestimated in most of the models24,26,27,28,29,30. For example, the concentration of free Ca2+ in the ER is typically in the range of a few hundred micromolars. Our model is capable of reproducing the observed magnitudes of [Ca2+]Cyt, [Ca2+]ER and [Ca2+]Mt.
In 2013, Szopa et al. suggested a model to explicitly take into account the existence of Ca2+ microdomain between the ER and mitochondria to discuss the mitochondrial Ca2+ uptake29. However, the assumption in the model that MCU directly senses the [Ca2+]ER may be not very appropriate, particularly in view of the existing sharp Ca2+ gradients in the microdomain against the distance between the IP3R and MCU. Previously, there are mainly two basic ways to handle Ca2+ microdomain in the whole-cell models. The first approach involved the separation of a specified small region from the cell. But this subspace is simply treated as another well-mixed compartment57,58 and thus this approach cannot capture the fact of sharp Ca2+ concentration gradients in the microdomain43,44. Sneyd et al. used an alternative approach to divide the cell into a few separate domains, connected by many boundary fluxes59,60. Although this is a powerful approach, it greatly increases the complexity of the model, leading to extreme computational difficulties.
Different from these methods, a new approach has been suggested in the present paper. For simplicity, all the IP3Rs dynamics and SERCA are determined by the bulk [Ca2+]Cyt. As a result, the bulk Ca2+ concentrations in cytosol, ER and mitochondria can be examined in deterministic ODE which actually assuming that the cytosolic, ER and mitochondrial compartments are mixed homogenously. Upon such well mixed compartments, a microdomain is specifically considered to discuss the MCU dynamics which is crucial for mitochondrial Ca2+ uptake. The microdomain consists of a cluster of IP3Rs and a MCU separated at a short distance. Treated as a point release source for the clustered IP3Rs which dynamics is controlled by bulk [Ca2+]Cyt, a sharp distribution of [Ca2+]Mic can be built up in the microdomain. Each MCU responds to the local high [Ca2+]Mic generated by the clustered IP3Rs, rather than the bulk [Ca2+]Cyt. As a result, this method avoids the simulation of the Ca2+ diffusion in microdomain between the ER membrane and IMM.
Peglow et al.30 discussed a three-dimensional model to analyze the influence of the distance between mitochondria and the CRAC channel on the plasma membrane, which is responsible for Ca2+ influx from the outside of the cell, on global Ca2+ oscillation. They confirmed that the relative position between mitochondria and the CRAC channels controls the global Ca2+ signal. Their simulation results showed that close mitochondria will lower the microdomain [Ca2+] right at the CRAC channels and prevent the Ca2+-dependent inactivation of CRAC channels, leading to higher Ca2+ influx and thereby increasing global [Ca2+]Cyt. They found that the global [Ca2+]Cyt monotonously changes with the mitochondria-CRAC distance from 0.1 to 2 μm. A similar result is obtained in our model at r > rcrt.
As a fact, our model does not realistically consider the spatial structure and so many important factors of Ca2+ signaling have been ignored. In the model the microdomain is modeled as a radially symmetric hemisphere and so the linearized reaction-diffusion equation has been applied to obtain a simple expression for [Ca2+]Mic. The mitochondrial boundary is actually ignored. One may expect that the mitochondrial boundary effect may modulate the sharp [Ca2+]Mic distribution. The Ca2+ release from the clustered IP3Rs are treated as the point release source, which is correct for far away MCU. But if MCU is too close to IP3Rs, a better treatment is to consider the spatial distribution of IP3Rs. The model also assumes that OMM has no effect on the Ca2+ diffusion as it has high permeability to Ca2+. A better MCU model should also consider the regulation effect of MICU2 on the MCU channel activity. Our model is primarily designed for non-excitable cells, i.e. a ‘closed’ cell model, ignoring the Ca2+ exchange between cytosol and extracellular medium through the plasma membrane. Thus, a more realistic Ca2+ model should be considered in the future. However, despite oversimplification in some details, we believe that the present model is useful and may provide us some insights on how the crosstalk between the ER and mitochondria modulates Ca2+ signals in living cells.
References
Berridge, M. J., Bootman, M. D. & Roderick, H. L. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Bio. 4, 517–529 (2003).
Parekh, A. B. Decoding cytosolic Ca2+ oscillations. Trends Biochem. Sci. 36, 78–87 (2011).
Contreras, L., Drago, I., Zampese, E. & Pozzan, T. Mitochondria: the calcium connection. BBA-Bioenergetics 1797, 607–618 (2010).
Rizzuto, R., Brini, M., Murgia, M. & Pozzan, T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science 262, 744–747 (1993).
Hajnóczky, G., Robb-Gaspers, L. D., Seitz, M. B. & Thomas, A. P. Decoding of cytosolic calcium oscillations in the mitochondria. Cell 82, 415–424 (1995).
Rizzuto, R., De Stefani, D., Raffaello, A. & Mammucari, C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Bio. 13, 566–578 (2012).
Giacomello, M. et al. Ca2+ Hot Spots on the Mitochondrial Surface Are Generated by Ca2+ Mobilization from Stores, but Not by Activation of Store-Operated Ca2+ Channels. Mol. Cell 38, 280–290 (2010).
Csordás, G. et al. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol. Cell 39, 121–132 (2010).
Jouaville, L. S., Ichas, F., Holmuhamedov, E. L., Camacho, P. & Lechleiter, J. D. Synchronization of calcium waves by mitochondrial substrates in Xenopus laevis oocytes. Nature 377, 438–441 (1995).
Vay, L. et al. Modulation of Ca2+ release and Ca2+ oscillations in HeLa cells and fibroblasts by mitochondrial Ca2+ uniporter stimulation. J. Physiol. 580, 39–49 (2007).
Olson, M. L., Chalmers, S. & McCarron, J. G. Mitochondrial Ca2+ uptake increases Ca2+ release from inositol 1, 4, 5-trisphosphate receptor clusters in smooth muscle cells. J. Biol. Chem. 285, 2040–2050 (2010).
Boitier, E., Rea, R. & Duchen, M. R. Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. J. Cell Biol. 145, 795–808 (1999).
Hajnóczky, G., Hager, R. & Thomas, A. P. Mitochondria suppress local feedback activation of inositol 1, 4, 5-trisphosphate receptors by Ca2+. J. Biol. Chem. 274, 14157–14162 (1999).
Olson, M. L., Chalmers, S. & McCarron, J. G. Mitochondrial organization and Ca2+ uptake. Biochem. Soc. Trans. 40, 158–167 (2012).
Csordás, G. et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 174, 915–921 (2006).
de Brito, O. M. & Scorrano, L. An intimate liaison: spatial organization of the endoplasmic reticulum–mitochondria relationship. EMBO J. 29, 2715–2723 (2010).
Williams, G. S., Boyman, L., Chikando, A. C., Khairallah, R. J. & Lederer, W. Mitochondrial calcium uptake. Proceedings of the National Academy of Sciences 110, 10479–10486 (2013).
Meyer, T. & Stryer, L. Molecular model for receptor-stimulated calcium spiking. Proc. Nat. Acad. Sci.USA 85, 5051–5055 (1988).
Li, Y. X. & Rinzel, J. Equations for InsP3 Receptor-mediated [Ca2+]i Oscillations Derived from a Detailed Kinetic Model: A Hodgkin-Huxley Like Formalism. J. Theor. Biol. 166, 461–473 (1994).
Shuai, J. W. & Jung, P. Stochastic Properties of Ca2+ Release of Inositol 1, 4, 5-Trisphosphate Receptor Clusters. Biophys. J. 83, 87–97 (2002).
Shuai, J. W. & Jung, P. Optimal ion channel clustering for intracellular calcium signaling. Proc. Nat. Acad. Sci.USA 100, 506–510 (2003).
Rüdiger, S., Shuai, J. W. & Sokolov, I. Law of mass action, detailed balance and the modeling of calcium puffs. Phys. Rev. Lett. 105, 048103 (2010).
Rüdiger, S. Stochastic models of intracellular calcium signals. Phys. Rep. 534, 39–87 (2014).
Cortassa, S., Aon, M. A., Marbán, E., Winslow, R. L. & O'Rourke, B. An integrated model of cardiac mitochondrial energy metabolism and calcium dynamics. Biophys. J. 84, 2734–2755 (2003).
Dash, R. K., Qi, F. & Beard, D. A. A biophysically based mathematical model for the kinetics of mitochondrial calcium uniporter. Biophys. J. 96, 1318–1332 (2009).
Marhl, M., Haberichter, T., Brumen, M. & Heinrich, R. Complex calcium oscillations and the role of mitochondria and cytosolic proteins. Biosystems 57, 75–86 (2000).
Fall, C. P & Keizer, J. E. Mitochondrial Modulation of Intracellular Ca2+ Signaling. J. Theor. Biol. 210, 151–165 (2001).
Ndiaye, D. et al. Characterization of the Effect of the Mitochondrial Protein Hint2 on Intracellular Ca2+ dynamics. Biophys. J. 105, 1268–1275 (2013).
Szopa, P., Dyzma, M. & Kazmierczak, B. Membrane associated complexes in calcium dynamics modelling. Phys. Biol. 10, 035004 (2013).
Peglow, M., Niemeyer, B. A., Hoth, M. & Rieger, H. Interplay of channels, pumps and organelle location in calcium microdomain formation. New J. Phys. 15, 055022 (2013).
Moreau, B., Nelson, C. & Parekhm, A. B. Biphasic regulation of mitochondrial Ca2+ uptake by cytosolic Ca2+ concentration. Curr. Biol. 16, 1672–1677 (2006).
Tang, S. et al. Design and application of a class of sensors to monitor Ca2+ dynamics in high Ca2+ concentration cellular compartments. Proc. Nat. Acad. Sci.USA 108, 16265–16270 (2011).
Csordás, G. et al. MICU1 Controls Both the Threshold and Cooperative Activation of the Mitochondrial Ca2+ Uniporter. Cell Metab. 17, 976–987 (2013).
McCarron, J. G., Olson, M. L. & Chalmers, S. Mitochondrial regulation of cytosolic Ca2+ signals in smooth muscle. Pflügers Arch.,-EJP 464, 51–62 (2012).
Gunter, T. E. & Sheu, S. S. Characteristics and possible functions of mitochondrial Ca2+ transport mechanisms. BBA-Bioenergetics 1787, 1291–1308 (2009).
Mallilankaraman, K. et al. MICU1 Is an Essential Gatekeeper for MCU-Mediated Mitochondrial Ca2+ Uptake that Regulates Cell Survival. Cell 151, 630–644 (2012).
Baughman, J. M. et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476, 341–345 (2011).
De Stefani, D., Raffaello, A., Teardo, E., Szabò, I. & Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476, 336–340 (2011).
Raffaello, A. et al. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J. 32, 2362–2376 (2013).
Csordás, G., Thomas, A. P. & Hajnóczky, G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J. 18, 96–108 (1999).
Taufiq-Ur-Rahman, A. S., Falcke, M. & Taylor, C. W. Clustering of InsP3 receptors by InsP3 retunes their regulation by InsP3 and Ca2+. Nature 458, 655–659 (2009).
Dickinson, G. D., Swaminathan, D. & Parker, I. The probability of triggering calcium puffs is linearly related to the number of inositol trisphosphate receptors in a cluster. Biophys. J. 102, 1826–1836 (2012).
Vais, H., Foskett, J. K. & Mak, D.-O. D. Unitary Ca2+ current through recombinant type 3 InsP3 receptor channels under physiological ionic conditions. J. Gen. Physiol. 136, 687–700 (2010).
Smith, G. D. Analytical steady-state solution to the rapid buffering approximation near an open Ca2+ channel. Biophys. J. 71, 3064–3072 (1996).
Neher, E. Vesicle Pools and Ca2+ Microdomains: New Tools for Understanding Their Roles in Neurotransmitter Release. Neuron 20, 389–399 (1998).
Arnaudeau, S., Kelley, W. L., Walsh, J. V. & Demaurex, N. Mitochondria recycle Ca2+ to the endoplasmic reticulum and prevent the depletion of neighboring endoplasmic reticulum regions. J. Biol. Chem. 276, 29430–29439 (2001).
Ishii, K., Hirose, K. & Iino, M. Ca2+ shuttling between endoplasmic reticulum and mitochondria underlying Ca2+ oscillations. EMBO Rep. 7, 390–396 (2006).
Kornmann, B. The molecular hug between the ER and the mitochondria. Curr. Opin. Cell Biol. 25, 1–6 (2013).
Berridge, M. J. Calcium oscillations. J. Biol. Chem. 265, 9583–9586 (1990).
Camacho, P. & Lechleiter, J. D. Increased frequency of calcium waves in Xenopus laevis oocytes that express a calcium-ATPase. Science 260, 226–229 (1993).
Kim, I., Xu, W. J. & Reed, J. C. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 7, 1013–1030 (2008).
Kaftan, E. J., Xu, T., Abercrombie, R. F. & Hille, B. Mitochondria shape hormonally induced cytoplasmic calcium oscillations and modulate exocytosis. J. Biol. Chem. 275, 25465–25470 (2000).
Pacher, P., Csordás, G., Schneider, T. G. & Hajnóczky, G. Quantification of calcium signal transmission from sarco-endoplasmic reticulum to the mitochondria. Journal Physiol. 529, 553–564 (2000).
Wang, X. & Schwarz, T. L. The Mechanism of Ca2+-Dependent Regulation of Kinesin-Mediated Mitochondrial Motility. Cell 136, 163–174 (2009).
Friedman, J. R., Lackner, L. L, West, M., DiBenedetto, J. R., Nunnari, J. & Voeltz, G. K. ER tubules mark sites of mitochondrial division. Science 334, 358–362 (2011).
Walther, P., Höhn, K. & Krisp, H. What is the true size of the mitochondrial intermembrane space? A study using high-pressure freezing and STEM tomography. Proceedings of MC 2, 49–50 (2009).
Jafri, M. S., Rice, J. J. & Winslow, R. L. Cardiac Ca2+ dynamics: The roles of ryanodine receptor adaptation and sarcoplasmic reticulum load. Biophys. J. 74, 1149–1168 (1998).
LeBeau, A. P., Van Goor, F., Stojilkovic, S. S. & Sherman A. Modeling of membrane excitability in gonadotropin-releasing hormone-secreting hypothalamic neurons regulated by Ca2+ mobilizing and adenylyl cyclase-coupled receptors. J. Neurosci. 20, 9290–9297 (2000).
Higgins, E. R. et al. Modelling calcium microdomains using homogenisation. J. Theor. Biol. 247, 623–644 (2007).
Means, S. A. & Sneyd, J. Spatio-temporal calcium dynamics in pacemaking units of the interstitial cells of Cajal. J. Theor. Biol. 267, 137–152 (2010).
Acknowledgements
We would like to acknowledge many useful conversations with George Dickinson, Ian Parker and Jeffery Lock in University of California, Irvine. We also thank Ke Jiang and Yu Jiang in Xiamen University for help with numerical simulations. This work was supported by the China National Funds for Distinguished Young Scholars under grant 11125419, the National Natural Science Foundation of China under grant 31370830, the Fujian Province Funds for Leading Scientist in Universities and ICAM branches cost sharing fund.
Author information
Authors and Affiliations
Contributions
H.Q. and J.S. conceived and designed the model. H.Q. performed the numerical simulations. L.L. contributed to the writing of the programs. H.Q. and J.S. wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Table
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Qi, H., Li, L. & Shuai, J. Optimal microdomain crosstalk between endoplasmic reticulum and mitochondria for Ca2+ oscillations. Sci Rep 5, 7984 (2015). https://doi.org/10.1038/srep07984
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07984
This article is cited by
-
Systems modeling predicts that mitochondria ER contact sites regulate the postsynaptic energy landscape
npj Systems Biology and Applications (2021)
-
Modeling the role of endoplasmic reticulum-mitochondria microdomains in calcium dynamics
Scientific Reports (2019)
-
The Endoplasmic Reticulum Stress Response in Neuroprogressive Diseases: Emerging Pathophysiological Role and Translational Implications
Molecular Neurobiology (2018)
-
High resolution structural evidence suggests the Sarcoplasmic Reticulum forms microdomains with Acidic Stores (lysosomes) in the heart
Scientific Reports (2017)
-
Regulation of 1, 4, 5-triphosphate receptor channel gating dynamics by mutant presenilin in Alzheimer’s disease cells
Frontiers of Physics (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.