-
PDF
- Split View
-
Views
-
Cite
Cite
Nathan Weisz, Christian Wienbruch, Katalin Dohrmann, Thomas Elbert, Neuromagnetic indicators of auditory cortical reorganization of tinnitus, Brain, Volume 128, Issue 11, November 2005, Pages 2722–2731, https://doi.org/10.1093/brain/awh588
Close - Share Icon Share
Abstract
Animal studies show that following damage to inner-ear receptors, central representations of intact lesion-edge (LE) frequencies become enlarged (map reorganization). One theory of tinnitus holds that this process could be related to the tinnitus sensation. To test this hypothesis, neuromagnetic evoked fields of tinnitus participants with high-frequency hearing loss and normal hearing controls were measured, while subjects listened to monaurally presented LE or control (CO; an octave below LE) tones. The predictions made based on the map reorganization hypothesis of tinnitus were that neuronal responses to LE frequencies would be enhanced, and that source localizations for LE would be distorted. N1m equivalent dipole moments for LE were not supranormal in the tinnitus group, whereas responses to CO of tinnitus patients compared to controls were enlarged in the right hemisphere. This effect was positively associated with tinnitus-related distress. Abnormal source locations were found for generators activated by LE tones in the right hemisphere of the tinnitus group. This right-hemispheric map distortion was not associated with subjective variables of tinnitus. A positive correlation with tinnitus distress was found for the left hemisphere with more anterior sources being associated with enhanced distress. However, this result was independent of the frequency of the stimulus. Overall, the present study suggests that mechanisms of map reorganization, although present in the data, cannot satisfactorily explain the emergence of tinnitus and that differential hemispheric involvement must be considered.
Introduction
Subjective tinnitus refers to the experience of a simple sound or sounds (e.g. pure tone, hissing, etc.) without a detectable physical source. Approximately 10% (Bauer, 2004) of the population experience this sensation permanently, and for some (∼1–3% of the population) this condition can adversely affect everyday living. In most cases, tinnitus is accompanied by an audiometrically measurable hearing loss, and even in a majority of those cases with normal audiograms abnormal outer hair-cell functioning has been reported (Shiomi et al., 1997). Based on this evidence, it seems that some damage to the hearing system, even if temporary, is an essential prerequisite for the development of tinnitus (Feldmann, 1992; Lockwood et al., 2002; Eggermont, 2003). However, notions that tinnitus is generated by the peripheral auditory system had to be discarded (Kemp, 1981; Douek, 1987; Møller, 2003). It is now widely assumed that peripheral damage triggers changes in the central auditory system (Mühlnickel et al., 1998; Diesch et al., 2004), which are then interpreted as tinnitus by the higher processing stages in the brain (Jastreboff, 1990). Much neuroscientific tinnitus research has focused on measures of spontaneous activity, e.g. in the dorsal cochlear nucleus, inferior colliculus or the auditory cortex (Sasaki et al., 1981; Brozoski et al., 2002; Norena and Eggermont, 2003), which assumed tinnitus to be a consequence of hyperactivity (Kaltenbach and Afman, 2000; Kaltenbach et al., 2004) and enhanced synchronization of neuronal activity (Ochi and Eggermont, 1997; Norena and Eggermont, 2003). Another theory of tinnitus views it mainly as a consequence of map reorganization (Mühlnickel et al., 1998; Lockwood et al., 2002; Møller, 2003), analogous to changes observed in somatosensory phantom-limb pain following amputation (Elbert et al., 1994; Flor et al., 1995, 1998). Although different structurally, similar underlying processes are discussed as being responsible for changes in spontaneous activity and map organization: a reduction of intracortical inhibition due to the afferent deprivation following a receptor damage (Eggermont and Roberts, 2004).
The aim of the present study was to test the relevance of map reorganization and tinnitus onset. The appeal of this hypothesis stems not only from a descriptive level, i.e. the parallel presence of deafferentation and phantom perceptions, but also from a relationship between these two phenomena in the somatosensory modality. In the case of phantom-limb pain, very strong correlations were found between the extent of map reorganization in deafferented cortex and the strength of subjectively reported pain (Flor et al., 1995; Elbert et al., 1998). In this work, map reorganization was defined as the extent to which the source of the region neighbouring the deprived area (i.e. contralateral to the amputated limb) on the somatotopic map, so-called lesion-edge (LE) areas, has invaded the representational zones deprived of its input. We attempted to apply a similar approach with tinnitus.
A previous study (Mühlnickel et al., 1998) by members of our group investigated the representation of the ‘tinnitus-frequency’ rather than the LE, and found distorted locations of this frequency to be correlated with the reported tinnitus strength. However, audiometrically impaired subjects, i.e. typical tinnitus sufferers, were excluded from the study. More recent data by Norena et al. (2002) show that tinnitus subjects rate an extremely wide range of frequencies (ratings reflect often a ‘mirror-image’ of the audiogram) as similar to their tinnitus sound, even with those subjects who report hearing only one sound. In the light of this data, the entire concept of ‘tinnitus-frequency’ is debatable and it seems more meaningful to design studies on more valid measures such as hearing loss and tinnitus sensation. A considerable bilateral expansion of the tonotopic map has been observed in blind individuals (Elbert et al., 2002). As these subjects did not report tinnitus, this raises further questions about the causal relationship between map reorganization and tinnitus.
A consistent finding from animal studies, following experimentally induced cochlear damage, is that receptive fields of deafferented neurons shift towards undamaged LE receptors (Rajan and Irvine, 1998; Rauschecker, 1999; Irvine et al., 2001). An analysis of the event related potentials/fields elicited by LE stimuli using a larger cortical patch (and, subsequently, more neurons involved in the processing of LE frequencies) should yield distinct differences between tinnitus sufferers and normal hearing controls. Indeed, this is what Dietrich et al. (2001) found for source strength of the N1m: responses to audiometric edge frequencies were significantly enhanced in subjects with high-frequency hearing loss and tinnitus, as compared to audiometrically normal frequencies far from the edge frequency. However, source localization data was not reported. An enhanced representation of LE frequencies seems also to be reflected on a perceptual level. Thai-Van et al. (2002, 2003) were able to show ameliorated frequency discrimination capabilities of subjects with high frequency hearing loss at the audiometric edge. This finding motivated an earlier EEG study by our group (Weisz et al., 2004b), in which an abnormal auditory mismatch response pattern in a time window ∼100 ms post-stimulus onset, restricted to the LE region was found. This result correlated strongly with the amount of tinnitus-related distress and source localization in the posterior–anterior direction was positively associated with the amount of distress. The interindividual variability spanned almost 5 cm, implicating that sources outside of the auditory cortex were also involved. It was not possible to discern from these results how much reorganization within the auditory cortex contributed to the findings.
In the present study, we used a montage of several regional sources (acting as a spatial filter) to investigate whether tinnitus is accompanied by deviant source localizations of LE frequencies on the three spatial gradients (posterior–anterior, medial–lateral, inferior–superior). Because the neuromagnetic investigation of tonotopic representation in humans is consistent on a group level (Romani et al., 1982; Pantev et al., 1995; Weisz et al., 2004a, c), yet highly variable on an individual level (Lütkenhöner et al., 2003; Weisz et al., 2004c), we additionally assessed tonotopy via the 2D Euclidean distance between the LE frequency and a frequency of an octave below LE (CO; i.e. well in the normal hearing range) taking into account the source locations on the two main tonotopic gradients (posterior–anterior and medial–lateral). We also tested whether the strength of the N1m response showed abnormalities as previously reported by Dietrich et al. (2001). Neurophysiological variables were correlated with scores of tinnitus-related distress. Based on the animal data described above, we expected to find an enhanced distance between the LE and the CO representation for the tinnitus group, which could be interpreted as evidence for an altered tonotopic representation. For the phantom-limb analogy to qualify for tinnitus, these deviations should be correlated with the amount of tinnitus-related distress.
Materials and methods
Participants
In this study, 14 tinnitus (mean = 49.64 years, SE = 3.30) and 11 normal-hearing control (mean = 43.18 years, SE = 4.37) male subjects participated. All tinnitus subjects exhibited a moderate to severe high-frequency hearing loss (assessed with an InterAcoustics AC40 clinical audiometer). An overview of the tinnitus participants is provided in Table 1. Following an explanation of the study, written informed consent was obtained from each individual according to the Declaration of Helsinki. Tinnitus subjects also filled out a standard German questionnaire assessing tinnitus-related distress (Goebel and Hiller, 1998). The study was approved by the Konstanz University Ethical Board.
An overview of the tinnitus participants
| Age . | Aetiology . | T-side . | HL-left* . | HL-right* . | TF . |
|---|---|---|---|---|---|
| 70 | Unknown | Bilateral | 70 | 69 | 35 |
| 38 | Unknown | Left | 38 | 70 | 23 |
| 55 | Occupational noise | Right domain | 55 | 55 | 34 |
| 40 | Sudden hearing loss | Bilateral | 40 | 42 | 19 |
| 55 | Unknown; mandibular disorder | Left domain | 55 | 44 | 15 |
| 36 | Sudden hearing loss | Bilateral | 36 | 36 | 39 |
| 35 | Sudden hearing loss | Right | 35 | 37 | 20 |
| 40 | Noise trauma | Left | 40 | NA† | 5 |
| 68 | Noise trauma | Left | 68 | 52 | 67 |
| 44 | Sudden hearing loss | Left | 44 | 52 | 61 |
| 49 | Sudden hearing loss | Left | 49 | 46 | 29 |
| 48 | Sudden hearing loss | Bilateral | 48 | 44 | 51 |
| 53 | Sudden hearing loss | Bilateral | 53 | 55 | 16 |
| 55 | Unknown | Right | 55 | 50 | 22 |
| Age . | Aetiology . | T-side . | HL-left* . | HL-right* . | TF . |
|---|---|---|---|---|---|
| 70 | Unknown | Bilateral | 70 | 69 | 35 |
| 38 | Unknown | Left | 38 | 70 | 23 |
| 55 | Occupational noise | Right domain | 55 | 55 | 34 |
| 40 | Sudden hearing loss | Bilateral | 40 | 42 | 19 |
| 55 | Unknown; mandibular disorder | Left domain | 55 | 44 | 15 |
| 36 | Sudden hearing loss | Bilateral | 36 | 36 | 39 |
| 35 | Sudden hearing loss | Right | 35 | 37 | 20 |
| 40 | Noise trauma | Left | 40 | NA† | 5 |
| 68 | Noise trauma | Left | 68 | 52 | 67 |
| 44 | Sudden hearing loss | Left | 44 | 52 | 61 |
| 49 | Sudden hearing loss | Left | 49 | 46 | 29 |
| 48 | Sudden hearing loss | Bilateral | 48 | 44 | 51 |
| 53 | Sudden hearing loss | Bilateral | 53 | 55 | 16 |
| 55 | Unknown | Right | 55 | 50 | 22 |
Hearing loss measured in dB HL.
Value not determined.
An overview of the tinnitus participants
| Age . | Aetiology . | T-side . | HL-left* . | HL-right* . | TF . |
|---|---|---|---|---|---|
| 70 | Unknown | Bilateral | 70 | 69 | 35 |
| 38 | Unknown | Left | 38 | 70 | 23 |
| 55 | Occupational noise | Right domain | 55 | 55 | 34 |
| 40 | Sudden hearing loss | Bilateral | 40 | 42 | 19 |
| 55 | Unknown; mandibular disorder | Left domain | 55 | 44 | 15 |
| 36 | Sudden hearing loss | Bilateral | 36 | 36 | 39 |
| 35 | Sudden hearing loss | Right | 35 | 37 | 20 |
| 40 | Noise trauma | Left | 40 | NA† | 5 |
| 68 | Noise trauma | Left | 68 | 52 | 67 |
| 44 | Sudden hearing loss | Left | 44 | 52 | 61 |
| 49 | Sudden hearing loss | Left | 49 | 46 | 29 |
| 48 | Sudden hearing loss | Bilateral | 48 | 44 | 51 |
| 53 | Sudden hearing loss | Bilateral | 53 | 55 | 16 |
| 55 | Unknown | Right | 55 | 50 | 22 |
| Age . | Aetiology . | T-side . | HL-left* . | HL-right* . | TF . |
|---|---|---|---|---|---|
| 70 | Unknown | Bilateral | 70 | 69 | 35 |
| 38 | Unknown | Left | 38 | 70 | 23 |
| 55 | Occupational noise | Right domain | 55 | 55 | 34 |
| 40 | Sudden hearing loss | Bilateral | 40 | 42 | 19 |
| 55 | Unknown; mandibular disorder | Left domain | 55 | 44 | 15 |
| 36 | Sudden hearing loss | Bilateral | 36 | 36 | 39 |
| 35 | Sudden hearing loss | Right | 35 | 37 | 20 |
| 40 | Noise trauma | Left | 40 | NA† | 5 |
| 68 | Noise trauma | Left | 68 | 52 | 67 |
| 44 | Sudden hearing loss | Left | 44 | 52 | 61 |
| 49 | Sudden hearing loss | Left | 49 | 46 | 29 |
| 48 | Sudden hearing loss | Bilateral | 48 | 44 | 51 |
| 53 | Sudden hearing loss | Bilateral | 53 | 55 | 16 |
| 55 | Unknown | Right | 55 | 50 | 22 |
Hearing loss measured in dB HL.
Value not determined.
Procedure
Pure tones (50 dB SL; 300 ms; 10 ms rise/fall) were presented monaurally via plastic tubes with an ITI varying between 2 and 2.5 s. For tinnitus participants, two frequencies were chosen according to the audiogram: (i) the LE frequency resembled the audiometrically normal edge frequency of the hearing loss slope and (ii) the CO frequency was set at an octave below LE. Control subjects were matched with tinnitus patients based on age and the same stimulus set was presented to both subjects. Blocks of conditions (300 tones per condition) were presented for both ears separately in a random order, resulting in a total of four runs per subject.
Data acquisition
Neuromagnetic data were recorded (A/D conversion rate: 678.17 Hz; 0.1–200 Hz bandpass) with a 148 channel whole-head magnetometer (4D Neuroimaging Inc., San Diego, CA). Vertical and horizontal electro-oculograms were measured from above and below the eye and from the outer canthi in order to detect epochs contaminated by eye-movements and eye-blinks.
Data analysis
To correct for artefacts caused by eye-movements, we used the multiple source eye correction method developed by Berg and Scherg (1994). For each condition, 300 epochs of 700 ms length (200 ms pre-stimulus baseline) were extracted from the continuous data. Epochs still contaminated by artefacts were rejected by visual inspection using the artefact scanning tool of BESA. The remaining epochs were averaged and 1–20 Hz bandpass filtered. The goal of source analysis was 2-fold: (i) to detect possible spatial deviations from normal tonotopic representation and (ii) to determine the neuronal response strengths for each condition. Therefore, a montage consisting of eight regional sources was created, two of which were placed symmetrically and bilaterally in the auditory cortex. The purpose of this measure was to ensure that the two sources of interest (i.e. the auditory ones) did not pick up too much activation from surrounding sources, i.e. the other six dipoles acted as a kind of spatial filter. A time window of 30 ms around the peak of the most prominent response was used for source analysis (N1m). Because any sound activated both hemispheres, two auditory sources were fitted simultaneously to the observed data with the only constraint being that they were symmetric sound (spherical volume conductor with single homogeneous shell). This was undertaken to minimize distorting effects of potentially strong ipsilateral effects on the source of interest (i.e. the contralateral one). Fixed locations of the auditory dipoles were used for all subjects and the orientations adjusted individually for all conditions (according to maximum amplitude) to analyse dipole strength.
Source strength is reported for orientation 1 only, since no substantial activation could be found for orientation 2 (meaning that the N1m is very well defined by one single tangential dipole in the auditory cortex). Because the main intention of the study is to investigate group × condition interactions, we decided to scale the data of the individual with the square root of the sum of squares of all values divided by the number of values minus one (root mean square). This was done to deal with the individual determination of frequencies for each participant, which poses as a source of variance especially for source location and strength potentially masking effects. Inference statistics are reported only for the scaled data given that there is no single measure that resulted in a group main effect.
For our purposes, a rather liberal operationalization of tonotopic representation was sufficient. It was assumed that if different frequencies are processed in spatially different neuronal units, then there would be a difference between these points. Tonotopy was thus investigated on all three spatial gradients (medial–lateral, posterior–anterior and inferior–superior) and additionally operationalized as the Euclidean distance between the locations for LE and CO in the 2D axial plane spanned by the source locations on the medial–lateral and posterior–anterior axes (i.e. the main tonotopic gradients). The logarithm of the Euclidean distance values was computed before running statistical analyses in order to provide a more normal distribution of the data. More positive values thus indicate an increased distance between CO and LE, and an expanded tonotopic representation.
Statistical analyses were conducted using a mixed-effects model (Pinheiro and Bates, 2000) package of R (Ihaka and Gentleman, 1996). This highly flexible statistical modelling technique allows not only for the formulation of a fixed effects part (population effects), but also for the specification of random effects, i.e. those associated with the individual experimental units. Significant interaction effects were broken down into more simple models.
Results
N1m
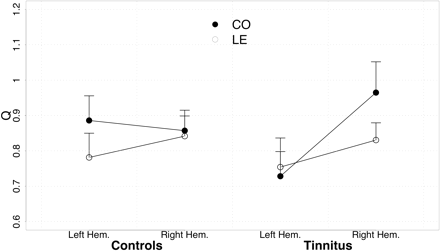
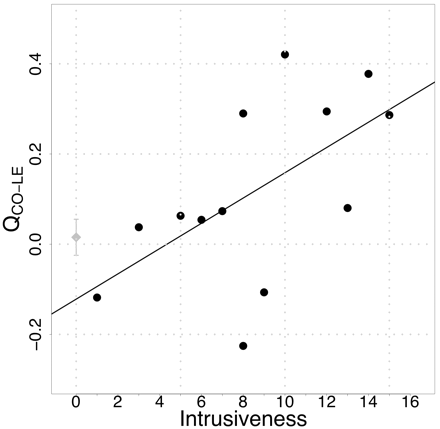
Peak latency for the N1m (seeFig. 1A for representative subject) was reached at 97.28 ms in control and 101.12 ms in tinnitus subjects. This group difference was not statistically significant (F1,23 = 1.36, n.s.). CO tones peaked somewhat later than LE tones (100.32 ms versus 98.55 ms; F1,23 = 6.60, P < 0.02). No significant associations with distress were found for this variable. Control subjects produced similar activations of source strength and hemispheric asymmetries for CO and LE tones in the left hemisphere (14.1 versus 14.4 nAm) and in the right hemisphere (13.7 versus 13.2 nAm). In contrast, tinnitus participants displayed enlarged responses to CO in the right hemisphere (19.0 versus 16.5 nAm), but not in the left hemisphere (13.1 versus 12.9 nAm). This pattern is more clear in the normalized data (Fig. 2), leading to a significant group × condition × hemisphere effect (F1,46 = 5.91, P < 0.02). When analysing the groups separately, a condition × hemisphere effect seems to be present in tinnitus subjects but not in controls (condition × hemisphere: F1,26 = 4.09, P < 0.053; in controls: F1,20 = 2.51, P < 0.13). The impression of a reversed hemispheric dominance for the two groups is strengthened when subtracting the activation to LE tones from those to CO tones (group × hemisphere: F1,23 = 8.83, P < 0.007). Whereas the difference is significantly enhanced (indicating stronger activation for CO) in the right hemisphere in tinnitus (F1,13 = 6.06, P < 0.03), it is in the opposite direction (i.e. left > right), yet not so pronounced, in controls (F1,10 = 3.84, P < 0.08). An association with tinnitus distress was found for this pattern (r = 0.55, P < 0.05) suggesting that the stronger CO relative to LE activation in the right hemisphere was correlated with distress. This seemed to be particularly true for the intrusiveness scale of the Tinnitus Fragebogen (r = 0.59, P < 0.04; seeFig. 3).
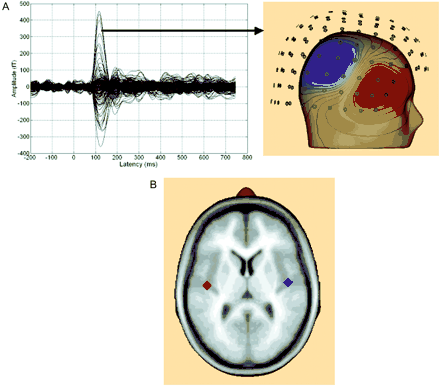
(A) The left panel shows an overlay of the evoked auditory field from the 148 channels recorded from a representative subject after left-ear stimulation. A clear N1m can be seen ∼120 ms post-stimulus onset. The distribution of the magnetic field over right sensors shows a pronounced dipolar pattern (right panel). (B) Overlay of the mean source locations fitted to the N1m for the left and right hemisphere shows generators located on Heschl's gyrus.
Source strength for auditory cortex dipoles. On average, tinnitus subjects exhibited stronger responses for CO in the right hemisphere.
The enhanced activation for CO as compared to LE in the right hemisphere is correlated with tinnitus-related distress, particularly tinnitus intrusiveness. The mean (±SE) for controls is indicated by the diamond on the y-axis.
Source localization
Medial–lateral
The frequently reported more medial location of higher frequencies could also be observed in the present data (CO = 51.35 mm; LE = 50.08 mm). Source locations in the right hemisphere were more lateral than in the left hemisphere (52.78 versus 48.66 mm; see alsoFig. 4; seeFig. 1b for overlay of mean source locations on standard brain). Analogously, the mixed-effects model analysis yielded significant condition (F1,46 = 5.61, P < 0.03) and hemisphere effects (F1,23 = 28.34, P < 0.001). However, no difference between groups could be observed (all Fs < 1).
Source localization on the medial–lateral axis (left panel). No differences were found for this measure.
Posterior–anterior
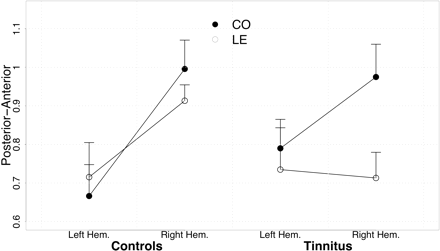
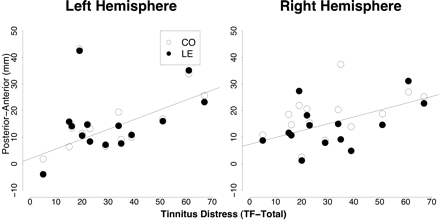
Sources were ∼1.4 mm further posterior for low frequencies than for LE (16.16 versus 17.54 mm; F1,46 = 5.93, P < 0.02). For both frequencies, they were 2 mm more anterior in the right hemisphere compared to the left (17.96 versus 15.88 mm, F1,23 = 5.09, P < 0.03). A significant condition × hemisphere effect (F1,46 = 4.84, P < 0.04) and a group × condition (F1,46 = 3.18, P < 0.09) is significant if one-sided testing is accepted. This is reasonable given that this effect was predicted. Indeed, it resulted in a distinct separation of CO and LE frequencies for the tinnitus subjects (F1,26 = 7.07, P < 0.02) whereas no such difference was obvious in controls: F1,20 = 0.12, P < 0.72; seeFig. 5). Right hemispheric activation in particular produced this tinnitus-related shift in LE versus CO source location, as suggested by Fig. 5: LE and CO locations are similar for both groups in the left hemisphere, whereas in the right hemisphere the LE location for tinnitus subjects deviates from control values. Associations with tinnitus-related distress were found for source localization on the posterior–anterior gradient for the left hemisphere (CO: r = 0.80, P < 0.001; LE: r = 0.74, P < 0.005). Sources were shifted in an anterior direction when distress levels were high. This pattern was also present in the right hemisphere (CO: r = 0.52, P < 0.07; LE: r = 0.63, P < 0.02; see alsoFig. 6).
Source location on the posterior–anterior axis shows almost identical values for the left hemisphere for the two groups. In comparison with the control group, locations for LE appear to be further posterior in the tinnitus group particularly in the right hemisphere.
A positive association between tinnitus-related distress and source location on the posterior–anterior axis was observed for the left hemisphere. Although not statistically significant, a similar trend was also observed for the right hemisphere (right panel). Note that this association appears to be independent of frequency.
Inferior–superior
Average location of sources on the inferior–superior axis ranged between 53 and 55 mm. Mixed-effects model analysis revealed no significant effects (all P > 0.1).
Euclidean distance
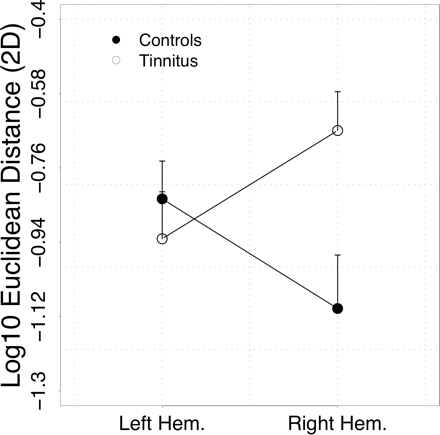
The analysis of the Euclidean distance in 2D space (posterior–anterior and medial–lateral) showed that distances between LE and CO frequencies are similar for the two groups in the left hemisphere (seeFig. 7). In tinnitus subjects, this distance is strongly enhanced in the right hemisphere as compared to controls. This pattern leads to a significant hemisphere × group interaction (F1,23 = 6.32, P < 0.02). Post hoc analysis indicates a significant group difference in the right hemisphere (F1,23 = 7.61, P < 0.02), while there is no indication of a difference in the left hemisphere (F1,23 = 0.40, n.s.). The associations with tinnitus-related distress (total score) were not significant in either hemisphere (left: r = −0.32, n.s.; right: r = −0.09, n.s.).
Tonotopic map distortions are particularly well captured by calculation of Euclidean distances in 2D space formed by the main tonotopic gradients (medial–lateral, anterior–posterior). While distances are almost the same in the left hemisphere, they are greatly enhanced in the right hemisphere of tinnitus subjects. Note that due to the calculation of the logarithm, less negative values indicate greater map distortions.
Discussion
In the present study, alterations in tonotopic representation and neuronal response dynamics to acoustic stimuli that might accompany tinnitus were investigated. Our approach differed from that taken by Mühlnickel et al. (1998), in which hearing loss was treated as a confounding factor and the focus was on individual ‘tinnitus frequency’. Hearing loss now forms an indispensable pillar in the manner in which we think about tinnitus. This is reflected in the present study design in which frequencies were chosen according to the individual audiogram, resembling the study done by Dietrich et al. (2001). These authors were able to show great enhancements of the N1m which were specific for the LE frequency, which fit data gained from animal studies nicely (Rajan and Irvine, 1998; Irvine et al., 2001). This result was not replicated in the present study. A possible reason for this discrepancy could lie in the different approach in determining the dipole moment. Dietrich et al. (2001) used the value gained from a single moving ECD while the present study applied a fixed source montage. It is known that source strength can vary considerably with depth of the dipole, which was extremely high on an average in the Dietrich et al. (2001) study, i.e. it is not known what influence source location might have played in the Dietrich et al. study since it was not reported.
The present study shows a greater response for CO in the right hemisphere of tinnitus subjects. This is in contrast to the finding of Dietrich et al. (2001). Furthermore, this pattern was associated with tinnitus-related distress, particularly intrusiveness, which assesses issues such as unpleasantness, perceived loudness and inability to concentrate due to the tinnitus. This result contradicts the phantom-limb pain analogy of tinnitus, which would predict such an association for enhanced representations of LE. It is possible that the finding reflects a greater salience of representation spared receptors as previously reported by Willott et al. (1994, 1996) for mice prepulse inhibition data.
One surprising finding was the strong lateralization of tinnitus-related effects, with right-sided deviances being prominent in terms of group differences. An intuitive assumption would be that this could be associated with the lateralization of tinnitus; a large proportion of our tinnitus subjects had left-sided (or dominant) tinnitus. However, 6 out of 8 subjects with non-left tinnitus (bilateral and right-sided) also showed greater activation for CO as compared to LE, just like the left-sided tinnitus sufferers (in this study, 5 out of 6). Although it cannot be excluded, it seems unlikely that tinnitus laterality played a major role. The standard model as detailed by Eggermont and Roberts (2004) is thus not capable to explain the present source strength data. This approach prefers to see the critical neuronal events leading to tinnitus as strictly contralateral to the damaged ear (see alsoLockwood et al., 1998). Why a large amount of tinnitus patients with bilateral hearing loss can have unilateral tinnitus (here 9/14) is not compatible with this framework either. Also, there seems to be a tendency in the tinnitus population to show left-sided tinnitus more often than right-sided (Axelsson and Ringdahl, 1989), which is again difficult to explain on the basis of the models and reasoning provided in the literature so far.
Concerning right hemispheric effects, Kang et al. (2003) reported an enhanced correlated metabolism in the right auditory cortex in bilaterally deaf subjects. It is thus possible that tinnitus laterality does not necessarily imply in which hemisphere tinnitus-relevant auditory cortex reorganization occurs, but rather that there could be an ear-independent differential hemispheric involvement in tinnitus. At this point, we may assume an increased predisposition of right auditory cortical neurons to synchronize their activity following deafferentation. Certainly, a model developed on such an assumption would have to eventually address the laterality issue. One admittedly speculative possibility could be a differential hemispheric involvement in processing auditory spatial information. In a recent fMRI study, Krumbholz et al. (2004) reported a greater left hemispheric sensitivity to right lateralized stimulation, whereas the right hemisphere was bilaterally sensitive. This could partially explain why our source strength effect, particularly the correlation with distress, is pronounced in the right hemisphere, even though the tinnitus is frequently perceived bilaterally.
Another measure showing a hemisphere effect is source localization in the anterior–posterior direction and also the 2D Euclidean distance measure. The tinnitus group exhibits an enhanced distance between LE and CO in the right hemisphere, which seems to be particularly driven (seeFig. 6) by a more posterior localization of LE. This also does not appear to be related to the side of cochlear impairment. Greater distances between LE and CO in the right than in the left hemisphere was shown in 3 out of 6 left-sided tinnitus patients and 8 out of 8 non-left tinnitus patients. From animal studies (Kaas et al., 1999; Kaas and Hackett, 2000), we know that the auditory cortex consists of several auditory fields of which many are tonotopically organized on an anterolateral–posteromedial gradient. In human, magnetic source imaging and intracortical EEG indicate that the generators for the N1m are mainly concentrated in the secondary auditory cortex, for which an anterolateral–posteromedial tonotopic gradient has been reported previously (Elberling et al., 1982; Pantev et al., 1988, 1995; Lütkenhöner and Steinstrater, 1998; Elbert et al., 2002; Weisz et al., 2004a). Higher frequencies are overall located more posteriorally and medially. Our finding is thus consistent in part with the map reorganization hypothesis (even though the hemispheric differences remain unresolved). However, differing from what we might expect from the phantom-limb pain analogy, the distance between LE and CO was not associated with the amount of tinnitus-related distress. However, in the left hemisphere, i.e. the one in which there is no convincing evidence for map reorganization, there was a very strong positive association between source location on the posterior–anterior gradient and tinnitus-related distress. Yet this correlation was equally strong for CO and LE. This finding, replicating that of an earlier study by our group (Weisz et al., 2004), is also difficult to reconcile with the map reorganization hypothesis, which would predict LE-specific effects. We assume that the condition independence is an indicator that sources outside of the secondary auditory cortex (largely responsible for the N1m in normal hearing subjects) are additionally active in highly distressed subjects. These could either be different auditory fields or regions outside of the auditory cortex, e.g. frontal regions.
A short comment on the limitations of this study seems appropriate. Even though the feasibility to investigate tonotopic organization using the N1m is a matter of strong ongoing discussion (Lütkenhöner et al., 2003), recent studies (Gabriel et al., 2004; Weisz et al., 2004a) corroborate gradients that were reported previously (Pantev et al., 1988, 1995; Lütkenhöner and Steinstrater, 1998; Elbert et al., 2002; for review seePantev and Lütkenhöner, 2000; the variability is however stronger in the more recent studies). Based on fMRI studies (Schönwiesner et al., 2002), showing the activation of multiple fields during auditory processing, it seems that the adequacy of fitting dipoles to model tonotopy can be generally called into question. Since the time resolution of fMRI is quite distant from the time scale of genuine neuronal activation, a one-to-one application of the fMRI critique to MEG seems inappropriate. Nevertheless, it is still likely that more than one auditory field is active in producing the N1m. Assuming that these fields have a similar spatial orientation (anterior–posterior, medial–lateral), it is still possible to talk about tonotopic gradients. One must remember that source models represent activity from several thousand synchronously activated neurons and therefore claims of localizing activity with high spatial resolution (within mm) must be made with caution. At the same time, when neurons are focally active, then fitting dipoles can be a good approximation of the centre of activational gravity. This seems to be the case with the N1, for which Godey et al. (2001) found identical localizations of activity between MEG and intracranial EEG in four epilepsy patients. Another possibility would be that different frequencies (e.g. 1000 versus 1500 Hz) totally change the pattern of which auditory fields become activated, an assumption difficult to justify theoretically and so far experimentally not proven.
The second limitation is related to the hearing loss of the tinnitus subjects. This partly reflects our view that representative subjective tinnitus is interwoven with hearing loss, as phantom-limb pain is with somatosensory deafferentation. But there is also a practical argument. Certainly, a potential control group for future studies would consist of subjects without tinnitus but with an equal pattern of hearing loss (also to exclude the unlikely possibility that our results reflect tinnitus-unspecific aspects). This may be difficult to attain given that hearing loss and tinnitus usually coexist. When tinnitus is properly assessed, such subjects seem to be rather rare. Other researchers report similar observations. Dietrich et al. (2001), for example, attempted to study cortical responses of subjects with high frequency hearing loss (similar to our subjects), but could not find a single person without tinnitus. Yet, finding an answer to the question why some persons with comparable audiological background as the ‘representative’ tinnitus sufferer do not develop such symptoms appears to be one of the most promising paths of unravelling some of the mysteries around tinnitus in general. In order to attain adequate group sizes calls for multicentric collaborations of tinnitus research groups employing identical experiment protocols.
Another important aspect is that although the term ‘lesion-edge’ is used in this article, it should be kept in mind that a clinical audiogram is a rather crude measure in assessing hearing damage (i.e. in the inner ear). Our approach is similar to other studies published (Dietrich et al., 2001; Thai-Van et al., 2004), but overall the LE determination should be seen as an approximation. It will definitely be rewarding in future to measure outer (e.g. distortion product otoacoustic emissions, widths of psychoacoustical tuning curves; Shiomi et al., 1997; Moore, 2002) or inner hair-cell function more directly (e.g. displacement of tip of tuning curve or the threshold equalizing noise test as a faster diagnostic; Moore et al., 2000). This should also contribute to the important question about the features of hearing loss leading to generation of tinnitus: Is, for example, a damage to outer hair cells enough, or does the development of tinnitus require also inner hair-cell damage (almost analogous to an amputation)? We infer from the current data that map reorganization can be observed particularly in the right hemisphere in subjects with hearing loss and tinnitus, but that there is little evidence for the phantom-limb pain analogy, i.e. for a causal relationship between map reorganization and tinnitus. However, this relationship cannot be completely excluded, as the N1m reflects mainly activity from the secondary auditory cortex. Some evidences indicate that some reorganizational changes take place in the primary auditory cortex (Lockwood et al., 1998; Norena and Eggermont, 2003; although this is not consistent, see alsoEggermont and Kenmochi, 1998; Mühlnickel et al., 1998), which does not necessarily imply secondary auditory changes. One possibility would be to apply the same logic of this study using steady-state (e.g. amplitude-modulated) tones, which have been reported to originate from the primary auditory cortex (Pantev et al., 1996). Something similar has been undertaken by Diesch et al. (2004), who found an increase of amplitudes when stimulated above the audiometric edge (the amplitude of the steady-state response is known to decrease with increasing carrier frequency; Ross et al., 2000; Weisz et al., 2004a). This could be an argument of increased synchrony in the deafferented areas, but it could also possibly reflect recruitment. Another finding was that tonotopic gradients were missing, which is also not in line with the map reorganization hypothesis as outlined above. The interpretation of the results of the Diesch study are however complicated by a missing control group. Further studies using steady-stimulation are needed to shed more light on the relationship between tinnitus and primary auditory cortex reorganization in humans. To conclude, possibly hearing loss sets off a cascade of neuroplastic alterations that include map reorganization and altered neural synchrony between the various hierarchical stages of the auditory information processing machine, but accumulating evidence points to the latter and not the former giving rise to the tinnitus sensations (Norena and Eggermont, 2003).
We thank Horst Böttcher from ProAkustik for providing a high-precision audiometer, Thomas Hartmann for support during data collection and Christina Robert for editing. This study was financed by a grant of the Deutsche Forschungsgemeinschaft (DFG EL101/21).
References
Axelsson A, Ringdahl A. Tinnitus—a study of its prevalence and characteristics.
Berg P, Scherg M. A multiple source approach to the correction of eye artifacts.
Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus.
Diesch D, Struve M, Rupp A, Ritter S, Hülse M, Flor H. Enhancement of steady-state auditory evoked magnetic fields in tinnitus.
Dietrich V, Nieschalk M, Stoll W, Rajan R, Pantev C. Cortical reorganization in patients with high frequency cochlear hearing loss.
Douek E. Tinnitus following surgery. In: Feldmann H, editor. Third Int. Tinnitus Seminar. Münster: Harsch;
Eggermont JJ, Kenmochi M. Salicylate and quinine selectively increase spontaneous firing rates in secondary auditory cortex.
Elberling C, Bak C, Kofoed B, Lebech J, Saermark K. Auditory magnetic fields: source location and ‘tonotopical organization’ in the right hemisphere of the human brain.
Elbert T, Flor H, Birbaumer N, Knecht S, Hampson S, Larbig W, et al. Extensive reorganization of the somatosensory cortex in adult humans after nervous system injury.
Elbert T, Candia V, Altenmuller E, Rau H, Sterr A, Rockstroh B, et al. Alteration of digital representations in somatosensory cortex in focal hand dystonia.
Elbert T, Sterr A, Rockstroh B, Pantev C, Muller MM, Taub E. Expansion of the tonotopic area in the auditory cortex of the blind.
Feldmann H. Pathophysiologie des Tinnitus. In: Feldmann H, editor. Tinnitus. Stuttgart: Georg Thieme;
Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, et al. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation.
Flor H, Elbert T, Mühlnickel W, Pantev C, Wienbruch C, Taub E. Cortical reorganization and phantom phenomena in congenital and traumatic upper-extremity amputees.
Gabriel D, Veuillet E, Ragot R, Schwartz D, Ducorps A, Norena A, et al. Effect of stimulus frequency and stimulation site on the N1m response of the human auditory cortex.
Godey B, Schwartz D, de Graaf JB, Chauvel P, Liegeois-Chauvel C. Neuromagnetic source localization of auditory evoked fields and intracerebral evoked potentials: a comparison of data in the same patients.
Goebel G, Hiller W. Tinnitus-Fragebogen (TF): Ein Instrument zur Erfassung von Belastung und Schweregrad bei Tinnitus. Göttingen: Hogrefe,
Ihaka R, Gentleman R. R: a language for data analysis and graphics.
Irvine DR, Rajan R, Brown M. Injury- and use-related plasticity in adult auditory cortex.
Jastreboff PJ. Phantom auditory perception (tinnitus): mechanisms of generation and perception.
Kaas JH, Hackett TA. Subdivisions of auditory cortex and processing streams in primates.
Kaas JH, Hackett TA, Tramo MJ. Auditory processing in primate cerebral cortex.
Kaltenbach JA, Afman CE. Hyperactivity in the dorsal cochlear nucleus after intense sound exposure and its resemblance to tone-evoked activity: a physiological model for tinnitus.
Kaltenbach JA, Zacharek MA, Zhang J, Frederick S. Activity in the dorsal cochlear nucleus of hamsters previously tested for tinnitus following intense tone exposure.
Kang E, Lee DS, Lee JS, Kang H, Hwang CH, Oh SH, et al. Developmental hemispheric asymmetry of interregional metabolic correlation of the auditory cortex in deaf subjects.
Kemp DT. Physiologically active cochlear micromechanics—one source of tinnitus.
Krumbholz K, Schonwiesner M, von Cramon DY, Rubsamen R, Shah NJ, Zilles K, et al. Representation of interaural temporal information from left and right auditory space in the human planum temporale and inferior parietal lobe.
Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wack DS, Murphy BW. The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity.
Lütkenhöner B, Steinstrater O. High-precision neuromagnetic study of the functional organization of the human auditory cortex.
Lütkenhöner B, Krumbholz K, Seither-Preisler A. Studies of tonotopy based on wave N100 of the auditory evoked field are problematic.
Moore BC, Huss M, Vickers DA, Glasberg BR, Alcantara JI. A test for the diagnosis of dead regions in the cochlea.
Mühlnickel W, Elbert T, Taub E, Flor H. Reorganization of auditory cortex in tinnitus.
Norena AJ, Eggermont JJ. Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus.
Norena A, Micheyl C, Chery-Croze S, Collet L. Psychoacoustic characterization of the tinnitus spectrum: implications for the underlying mechanisms of tinnitus.
Ochi K, Eggermont JJ. Effects of quinine on neural activity in cat primary auditory cortex.
Pantev C, Lütkenhöner B. Magnetencephalographic studies of functional organization and plasticity of the human auditory cortex.
Pantev C, Roberts LE, Elbert T, Ross B, Wienbruch C. Tonotopic organization of the sources of human auditory steady-state responses.
Pantev C, Hoke M, Lehnertz K, Lütkenhoner B, Anogianakis G, Wittkowski W. Tonotopic organization of the human auditory cortex revealed by transient auditory evoked magnetic fields.
Pantev C, Bertrand O, Eulitz C, Verkindt C, Hampson S, Schuirer G, et al. Specific tonotopic organizations of different areas of human auditory cortex revealed by simultaneous magnetic and electric recordings.
Rajan R, Irvine DR. Neuronal responses across cortical field A1 in plasticity induced by peripheral auditory organ damage.
Rauschecker JP. Auditory cortical plasticity: a comparison with other sensory systems.
Romani GL, Williamson SJ, Kaufman L. Tonotopic organization of the human auditory cortex.
Ross B, Borgmann C, Draganova R, Roberts LE, Pantev C. A high-precision magnetencephalographic study of human auditory steady-state responses to amplitude-modulated tones.
Sasaki CT, Babitz L, Kauer JS. Tinnitus: development of a neurophysiologic correlate.
Shiomi Y, Tsuji J, Naito Y, Fujiki N, Yamamoto N. Characteristics of DPOAE audiogram in tinnitus patients.
Thai-Van H, Micheyl C, Norena A, Collet L. Local improvement in auditory frequency discrimination is associated with hearing-loss slope in subjects with cochlear damage.
Thai-Van H, Micheyl C, Moore BC, Collet L. Enhanced frequency discrimination near the hearing loss cut-off: a consequence of central auditory plasticity induced by cochlear damage?
Weisz N, Keil A, Wienbruch C, Hoffmeister S, Elbert T. One set of sounds, two tonotopic maps: exploring auditory cortex with amplitude-modulated tones.
Weisz N, Voss S, Berg P, Elbert T. Abnormal auditory mismatch response in tinnitus sufferers with high-frequency hearing loss is associated with subjective distress level.
Weisz N, Wienbruch C, Hoffmeister S, Elbert T. Tonotopic organization of the human auditory cortex probed with frequency-modulated tones.
Willott JF. Physiological plasticity in the auditory system and its possible relevance to hearing aid use, deprivation effects, and acclimatization.