-
PDF
- Split View
-
Views
-
Cite
Cite
M. Natasha Rajah, Mark D'Esposito, Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory, Brain, Volume 128, Issue 9, September 2005, Pages 1964–1983, https://doi.org/10.1093/brain/awh608
Close - Share Icon Share
Abstract
Several neuroimaging studies of cognitive ageing have found that age-related deficits in working memory (WM) and episodic memory abilities are related to changes in prefrontal cortex (PFC) function. Reviews of these neuroimaging studies have generally concluded that with age there is a reduction in the hemispheric specialization of cognitive function in the frontal lobes that may either be due to dedifferentiation of function, deficits in function and/or functional reorganization and compensation. Moreover, previous reviews have considered the PFC as homogeneous in function and have not taken into account the possibility that region specific changes in PFC function may occur with age. In the current review we performed a qualitative meta-analytic review of all the functional magnetic resonance imaging ageing studies and positron emission tomography ageing studies of WM and episodic memory that report PFC activation, to determine if any region-specific changes occur. The results indicated that in normal ageing distinct PFC regions exhibit different patterns of functional change, suggesting that age-related changes in PFC function are not homogeneous in nature. Specifically, we hypothesize that normal ageing is related to the differentiation of cortical function in a bilateral ventral PFC and deficits in function in right dorsal and anterior PFC. As a result of these changes, functional compensation in left dorsal and anterior PFC may occur. We hope that future studies will be conducted to either confirm or counter these hypotheses.
Introduction
Ageing is related to the deterioration of numerous biological systems and functions in the human body. The underlying cause of senescence has been investigated at numerous levels of analysis and has been attributed to a variety of possible factors: changes in cellular metabolism, cell structure, cell–matrix interactions, neurotransmitter systems and the rate and accuracy of DNA replication (Osiewacz and Hamann, 1997; Ladislas, 2000; Labat-Robert, 2001; Campisi, 2003; Troen, 2003; Cabeza et al., 2005). With the increasing availability of brain imaging technologies, such as blood-oxygen-level-dependent functional MRI (BOLD fMRI) and positron emission tomography (PET), much of the recent research on ageing has focused on investigating the relationship between age-related changes in brain structure/function and concomitant changes in cognitive/behavioural abilities (Gabrieli, 1996; Grady et al., 1995; Madden et al., 1999; Raz, 2000; Cabeza, 2002; Craik and Grady, 2002; Della-Maggiore et al., 2002; Grady, 2002; Reuter-Lorenz, 2002; Gazzaley and D'Esposito, 2003; Buckner, 2004; Park et al., 2004). Though it can be argued that the neuroimaging approach cannot inform us on the direct underlying cellular or physiological causes of senescence, due to the gross level of anatomic and functional changes that they measure; functional neuroimaging studies of cognition in healthy young adults indicate that these techniques provide valuable, non-invasive, methods for gaining insight into how regional changes in neural structure and function relate to cognition and behaviour (Cabeza and Nyberg, 2000; D'Esposito, 2000; Duncan and Owen, 2000; Rugg et al., 2002; Friston, 2005). Moreover, recent findings indicate that the signals measured by BOLD fMRI and PET techniques are coupled to ‘real’ changes in neural activity. Thus, if one is interested in understanding how age-related changes in cognition and behaviour may be related to gross changes in neural structure and function, then neuroimaging is a valid technique to use (Braver and Barch, 2002; Della-Maggiore et al., 2002; Grady, 2002; Reuter-Lorenz, 2002; Tisserand et al., 2002; Gazzaley and D'Esposito, 2003; Johnson et al., 2004; Raz et al., 2004; Cabeza et al., 2005).
Concerns with using functional imaging to examine age-related differences in brain function
However, when using BOLD fMRI and PET techniques to examine age differences in brain activity one must keep in mind that normal ageing affects the cerebrovascular system, which in turn affects the neurovascular coupling that is the basis of the signals measured by these techniques (D'Esposito et al., 2003). For example, the cerebrovascular changes observed in normal ageing have been shown to decrease the signal-to-noise ratio, the amplitude and the spatial extent of the BOLD response measured in the healthy elderly (Taoka et al., 1998; D'Esposito et al., 1999a, 2003; Hesselmann et al., 2001; Huettel et al., 2001). In addition, normal ageing has been associated with a lag in the time-to-peak of the BOLD response (Taoko et al., 1998); however, the overall shape of the BOLD response does not change with age (D'Esposito et al., 1999a; Huettel et al., 2001). Therefore, one must be careful in interpreting age-related changes in functional activity, as measured by fMRI and PET, since the observed age differences in brain activity may be confounded by group differences in cerebral vascular function. This is especially true when interpreting age-related deficits in brain activity since these deficits may not mean that elderly subjects exhibit deficits in regional neural activity, but may instead be due to changes in neurovascular coupling which in turn causes decreases in signal-to-noise ratio, signal amplitude or signal spatial extent.
Addressing confounds due to age-related differences in cerebral vascular function
Overall, researchers in the field of cognitive neuroscience of ageing have successfully used both statistical and experimental manipulations to control for the aforementioned confounds (Buckner et al., 2000; D'Esposito et al., 2003; Gazzaley and D'Esposito, 2003). For example, investigators generally do not test for between-group differences in task-related brain activity in functional neuroimaging studies of ageing. Instead, within-group differences in task-related activity are examined, followed by tests of group-by-task interactions (Hazlett et al., 1998; Madden et al., 1999; Reuter-Lorenz et al., 2000; Rypma and D'Esposito, 2000; Dolcos et al., 2002; Iidaka et al., 2002; Morcom et al., 2003). Investigators have also used multivariate statistics and brain-behaviour correlation methods to control for the confounding effects of age-related changes in neurovascular coupling (Cabeza et al., 1997; Madden et al., 1999, 2002; McIntosh et al., 1999; Anderson et al., 2000; Della-Maggiore et al., 2000; Grady et al., 2002; Schiavetto et al., 2002; Morcom et al., 2003; Lustig and Buckner, 2004). In addition, parametric experimental designs are helpful in dissociating age-related differences in brain activity that are related to the parametric changes in task performance and not to age differences in cerebral vasculature or other experimental confounds (i.e. motor flexibility or fatigue; Grady et al., 1999; Buckner et al., 2000; Gould et al., 2003). Therefore, by controlling for these possible confounds several functional neuroimaging studies have contributed valuable information to the field of cognitive ageing and how age-related changes in brain structure and function may be related to the cognitive and behavioural deficits that occur with age (Craik and Grady, 2002; Cabeza et al., 2005).
Theoretical issues in examining age-related changes in PFC function: functional compensation and dedifferentiation perspectives
One of the brain regions exhibiting a strong age-related change in structure and function, which also impacts cognition and behaviour, is the prefrontal cortex (PFC) (Lapidot, 1987; Morgan, 1987; Nielsen-Bohlman and Knight, 1995; de Brabander et al., 1998; Langley and Madden, 2000; Raz, 2000; West, 2000; Grachev and Apkarian, 2001; Braver and Barch, 2002; Cabeza, 2002; Craik and Grady, 2002; Della-Maggiore et al., 2002; Grady, 2002; Reuter-Lorenz, 2002; Tisserand et al., 2002; Uylings and de Brabander, 2002; Gazzaley and D'Esposito, 2003; Peters and Rosene, 2003; Buckner, 2004). For example, in vivo and post-mortem studies of humans and primates have shown that the strongest age-related cerebral cortical change is PFC grey matter and white matter reductions (Raz, 2000; Tisserand et al., 2002). In addition, functional neuroimaging studies have reported age-related changes in PFC activation and its functional connectivity with posterior cortical regions across a variety of cognitive tasks (Grady et al., 1994, 1999; Cabeza et al., 1997; Madden et al., 1999; McIntosh et al., 1999; Della-Maggiore et al., 2000; Rypma and D'Esposito, 2000; Schreursa et al., 2001; Iidaka et al., 2002). Behaviourally, the PFC is involved in a variety of cognitive operations, including: working memory (WM), episodic memory (EM), inhibition, monitoring, strategic organization and planning (Stuss and Knight, 2002). However, since memory impairment is one of the hallmarks of ageing, the majority of neuroimaging studies in this area have focused on age-related changes in PFC function during WM and EM task performance (Craik and Salthouse, 2000).
Age-related increases and decreases in activation during WM and EM tasks have been reported in a variety of PFC regions including the ventrolateral, dorsolateral and anterior PFC (Nielsen-Bohlman Knight, 1995; Trott et al., 1997; Grady et al., 1998; Madden et al., 1999; Reuter-Lorenz et al., 2000; Rypma et al., 2001; Cabeza, 2002; Wegesin et al., 2002; Morcom et al., 2003). Often, when these age-related changes in PFC activity are observed, the older subjects also perform poorer than young subjects on the WM and EM tasks used (Anderson et al., 2000; Cabeza et al., 2000; Rypma et al., 2001). However, even in the absence of behavioural differences, studies have reported WM-related and EM-related differences in PFC activation in young versus old subjects (Cabeza et al., 1997; Rypma and D'Esposito, 2000).
Reviews of the cognitive neuroscience literature of memory and ageing have generally considered cognitive mechanisms underlying age-related deficits in WM and EM and viewed the PFC as a homogeneous region (Li et al., 2001; Braver and Barch, 2002; Cabeza, 2002; Cabeza et al., 2002; Della-Maggiore et al., 2002; MacPherson et al., 2002; Reuter-Lorenz, 2002; Gazzaley and D'Esposito, 2003; Ramnani and Owen, 2004; Reuter-Lorenz, 2002). For example, Cabeza (2002) observed that there is reduced lateralized PFC activity across WM and EM tasks with age and proposed the hemispheric asymmetry reduction in old adults (HAROLD) model, which has been supported by subsequent experimental findings. However, this model does not address whether these laterality effects are specific to particular brain regions or common to all brain regions; including the PFC and its various subdivisions. Also, the HAROLD model does not specify whether the underlying neural mechanisms for age-related reductions in lateralized activity are due to functional compensation (Cabeza, 2002; Reuter-Lorenz et al., 2000, 2002), primary deficits in function (Lapidot, 1987; McDowell et al., 1994), dedifferentiation of function (Li et al., 2001; Lindenberger et al., 2001), or some combination of these mechanisms.
According to the functional compensation view, age-related decreases or absences in activation reflect deficits in brain function (Cabeza, 2002; Cabeza et al., 2002, 2004) and the concomitant increases in activation reflect either successful compensation for these deficits, when there are no age-differences in performance, and `attempted' compensation for these deficits, when there is an age-related decrement in performance (Cabeza, 2002; Cabeza et al., 2000; Grady, 2002; Grady et al., 1999; Madden et al., 1999; Reuter-Lorenz et al., 2000). It is unclear from a compensation perspective whether these compensatory activations reflect the recruitment of different regions and processes, which assumes that regional process-specificity does not change with age, or whether these changes reflect alterations in the processes mediated by the recruited regions, which assumes that as a result of neural plasticity, regional process-specificity changes with age.
Investigators have interpreted age-related changes in brain activation patterns to support both compensation perspectives. For example, in a PET study investigating age-related differences in visual memory for sine-wave gratings, McIntosh et al. (1999) found that older subjects performed equivalently to young subjects, but recruited a different neural network to perform the task. It was concluded that these changes reflected age-related reorganization of cortical function. In contrast, Cabeza et al. (2003, 2004) argue that if the production-monitoring distinction for respective left/right PFC activation during EM retrieval is true, then increased bilateral PFC activity in older subjects may reflect compensatory use of production processes when monitoring processes fail and vice versa. This conclusion assumes that regional process-specificity is maintained across lifespan.
According to the dedifferentiation view, age-related changes in functional activations reflect deficits in neurotransmission, which in turn cause decreases in signal-to-noise ratio and less distinct neural representations (Li et al., 2001). It follows, that decreases in activation reflect a deficit due to reductions in regional process-specificity. Increases in activity reflect generalized spreading of activity due to reduced specialization of function, which may or may not be compensatory. Therefore, this view suggests that overall there is no change in regional process-specificity in cortical function across lifespan, but that this specificity becomes more generalised with normal ageing.
Therefore, age-related changes in PFC function may be due to primary deficits in function, functional compensation (with or without reorganization of function) and dedifferentiation of function. The functional compensation and dedifferentiation perspectives both assume that there are primary deficits in function with age that precipitate functional compensation. However, the functional compensation perspective does not state precisely what neural changes precipitate deficits in function, whereas the dedifferentiation perspective does. According to the dedifferentiation perspective age-related deficits in function are due to deficits in neurotransmission resulting in noisier internal cortical representations (deficits in function). The functional compensation and dedifferentiation perspectives also differ in how they interpret age-related increases in PFC function. According to the former perspective age-related increases in PFC activity are compensatory. According to the latter perspective these age-related increases in activity may not always be compensatory, since age-related reductions in regional process-specificity may result in aberrant neural activity. In some cases this may benefit task performance (compensation) and in other cases it may be detrimental to task performance. Therefore, the dedifferentiation perspective does not neglect the possibility that age-related increases in PFC activity may be compensatory.
Structural and functional heterogeneity in the human PFC
One problem shared by both the functional compensation and dedifferentiation perspectives is that they treat the PFC as one homogeneous region. However, there is evidence that the PFC is neither structurally nor functionally homogeneous (Brodmann, 1909; Economo and Koskinas, 1925; Pandya and Yeterian, 1985; Petrides and Pandya, 1994; D'Esposito et al., 1999b; Cabeza and Nyberg, 2000; Duncan and Owen, 2000). The PFC in humans compromises most of the frontal lobes and is located rostral to the central sulcus, anterior to the Sylvian fissure and excludes primary and association motor cortices. Structurally, the PFC consists mostly of neocortex which has six cellular layers (Talairach and Tournoux, 1988). Traditionally, neuroscientists have subdivided the PFC into the following ‘classical’ regions based on gross anatomic markers: orbitofrontal cortex, dorsolateral PFC, ventrolateral PFC, anterior PFC and medial PFC (Luria, 1962; Milner and Petrides, 1984; Mesulam, 1986; Morecraft et al., 1993; Petrides and Pandya, 1999, 2002; Petrides et al., 2002; Ongur et al., 2003). However, variation in cytoarchitecture has also been used to define distinct structural regions within the PFC (Brodmann, 1909; Economo and Koskinas, 1925; Bonin and Bailey, 1947; Petrides and Pandya, 1994, 1999, 2002; Ongur et al., 2003). For example, by using the Nissl staining method and light microscope Brodmann (1909) examined laminar differences in the human PFC and defined the following cytoarchitectonic areas, referred to as Brodmann areas (BA): 8, 9, 10, 11, 12, 44, 45, 46 and 47. Additional cytoarchitectonic maps of human and primate frontal lobes have also been defined (Economo and Koskinas, 1925; Walker, 1940; Bonin and Bailey, 1947; Petrides and Pandya, 1994, 1999, 2002); however, the Brodmann map has been the most widely used in expressing functional and structural neuroimaging results of humans. The Brodmann map of the human PFC has been superimposed on the traditional subdivisions of the human PFC (see Fig. 1) and cognitive neuroscientists often use both methods when discussing structural subdivisions within the human PFC.
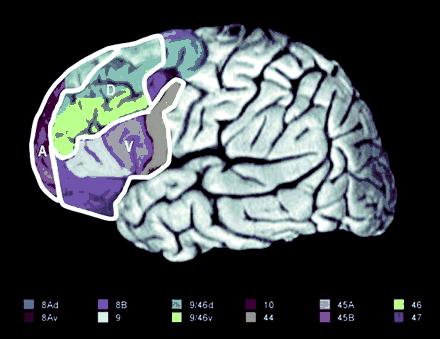
Diagram of the human prefrontal cortex (PFC; left lateral view). The ventral (V), dorsal (D) and anterior (A) subdivisions explored in this review are outlined in white. The Brodmann areas (BA) included in these subdivisions are each colourized separately. The legend for the colouring scheme is presented beneath the diagram. The ventral PFC included BAs 44, 45 and 47. The dorsal PFC included BA 9 (located on the middle frontal gyrus), BA 46 and BAs 9/46v and 9/46d (Petrides and Pandya, 1994, 1999). The anterior PFC included BAs 9 (located on the superior frontal gyrus) and 10 (Talairach and Tournoux, 1988). Figure reproduced with permission from Curtis and D'Esposito (2003).
Therefore, it is generally accepted that there is structural heterogeneity within the human PFC. Due to these structural differences it has also been suggested that there is functional heterogeneity in the roles played by distinct PFC regions in human cognition and behaviour. The arguments for functional heterogeneity of the PFC are supported by retrograde and anterograde tracer studies that have shown distinct patterns of cortico-cortical connectivity for dorsolateral, ventrolateral, orbitofrontal and medial PFC with posterior cortical regions (Carmichel and Price, 1998; Petrides and Pandya, 1999, 2002; Ongur and Price, 2000; Stefanacci and Amaral, 2002; Ongur et al., 2003). For example, Petrides and Pandya (1999, 2002) have shown that the dorsal and medial PFC regions are reciprocally connected with the posterior cortical regions involved in visuospatial processing, such as: superior parietal lobule, caudal portions of inferior parietal lobule, dorsal and medial occipital cortex, and caudal parietotemporal regions. In contrast, orbital and ventrolateral PFC regions are reciprocally connected with posterior cortical regions involved in object perception and identification, such as inferotemporal cortex. Therefore, it has been suggested that due to these different patterns of anatomical connectivity, the dorsal and medial areas of PFC are involved in visuospatial processing whereas the orbital and ventrolateral area of the PFC are involved in object meaning (Petrides and Pandya, 1999, 2002).
Evidence for the functional heterogeneity of the PFC also emerges from neuropsychological examinations of patients with frontal lobe damage (Petrides, 1985, 1996; Shimamura, 1995; Stuss and Alexander, 2000; Stuss et al., 2001; Mesulam, 2002; Stuss et al., 2002a; Fellows and Farah, 2003; Bechara, 2004; Roberts et al., 2004], lesion studies of non-human primates (Passingham, 1972, 1975; Mishkin, 1978; Mishkin and Manning, 1978; Dias et al., 1996, 1997; Collins et al., 1998; Petrides, 2000; Mesulam, 2002), physiological studies of regional differences in neurotransmitter modulation (Robbins, 2000; Castner et al., 2004; Seamans and Yang, 2004) and from neuroimaging studies examining cognitive function in healthy young adults (Cabeza and Nyberg, 2000; D'Esposito et al., 2000; Duncan and Owen, 2000; Rugg et al., 2002; Gilboa, 2004). For example, based on lesion studies of both human and non-human primates, Petrides (1994, 1996, 2002) has found that damage to the mid-ventrolateral PFC versus damage to the mid-dorsolateral PFC produces dissociable deficits on memory performance. Specifically, he argues that mid-ventrolateral PFC damage produces deficits in the active selection, comparison and judgement during retrieval in both WM and EM tasks (Petrides, 2002). In contrast, mid-dorsolateral PFC damage produces deficits in monitoring task performance during WM and EM tasks.
Similar dissociations in function have been observed in functional neuroimaging studies of WM and EM in healthy young adults (Cabeza and Nyberg, 2000; D'Esposito et al., 2000; Rugg et al., 2002, 2003; Wheeler and Buckner, 2003). For example, several studies have shown that manipulating information within WM activates the dorsolateral PFC whereas maintaining information within WM activates the ventrolateral PFC (D'Esposito, 1999b; 2000). EM studies have consistently reported left inferior PFC activity during encoding and bilateral dorsolateral and right anterior PFC activity during retrieval. In general, the focus of recent functional imaging studies has been on dissociating the functional contributions of the ventrolateral, dorsolateral and anterior PFC during EM and WM (Ranganath et al., 2000; Dobbins et al., 2003, 2004; Rugg et al., 2003). Therefore, in addition to structural heterogeneity, the neuropsychological and neuroimaging findings suggest that there is also functional heterogeneity between the ventral, dorsal and anterior PFC regions.
Examining region-specific changes in PFC function with age
We argue that neuroscience-based models of cognitive ageing must consider the above evidence that the PFC is neither structurally nor functionally homogeneous; especially since recent in vivo volumetric studies report that there are regional differences in age-related cortical atrophy. For example, studies have consistently reported volume reductions in lateral and orbital PFC with age (Raz et al., 1997; Salat et al., 2001; Tisserand, 2002, 2004). Given these reports of region-specific changes in the PFC structure with age, it is important that region-specific changes in PFC functions with age also be examined. In fact, several cognitive ageing studies have identified different patterns of activity and cortical atrophy in the ventral versus dorsal PFC across WM and EM tasks with age (Madden et al., 1999; Anderson et al., 2000; Cabeza et al., 2000; Raz, 2000; Schiavetto et al., 2002; Tisserand et al., 2002; Daselaar et al., 2003). However, to date, there has not been a review examining regionally-specific patterns of age-related changes in PFC function. Moreover, previous reviews have not considered the possibility that with age, distinct PFC regions may differentially reflect primary deficits in function, dedifferentiation of function and functional compensation with or without functional reorganization, respectively. These are the goals of the current review.
Unlike previous reviews of this literature, we have systematically tabulated age-related functional changes within distinct PFC regions, across tasks, to test the aforementioned hypotheses. Since the majority of the neuroimaging studies on cognitive ageing have utilized WM and EM tasks, we focus on these studies. We perform a qualitative meta-analytic review of all fMRI and PET studies archived by PubMed in the area of ageing, WM and EM that report PFC activation. Our goal is to examine age-related changes in the functional contributions of the ventral, dorsal and anterior PFC. We focus on these three subdivisions of the PFC since there is evidence that they are structurally distinct and also functionally distinct, within the context of WM and EM tasks (Petrides and Pandya, 1999, 2002; Ranganath and Paller, 1999; Cabeza and Nyberg, 2000; D'Esposito et al., 2000; Ranganath et al., 2000; Dobbins et al., 2002; Mesulam, 2002; Petrides et al., 2002; Rugg et al., 2002; Stuss et al., 2002). We predict that there will be a consistent pattern of region-specific, age-related, changes in PFC function across studies, which supports the hypothesis that ageing differentially affects distinct regions of PFC. In addition, we present some tentative predictions as to what mechanisms may cause these distinct regional changes: dedifferentiation of cortical function, deficits in cortical function or functional compensation, respectively. We hope our review will generate new hypotheses that stimulate future research in the cognitive neuroscience of ageing.
Methods
Inclusion criteria
We conducted a literature search using the keywords: ageing/age, brain, memory and imaging in PubMed for papers published between 1997 and the first half of 2004. Only studies that examined either the encoding phase, the retrieval phase, or both, in WM or EM were included in this review. Studies reviewed used either univariate or multivariate methods to analyse the imaging data. Only those studies that either used only within group analysis of young and older subjects, or, used both within and between group analyses, were included in this review.
Behavioural performance of the older subjects, relative to young, was not used to rule out study inclusion. Thus, in some studies older subjects performed equivalently to young in accuracy, whereas in others there was an age-related deficit in accuracy. Table 1 lists all 22 studies that were reviewed, the neuroimaging technique employed, a summary of the type of task employed in each study and the between-group behavioural accuracy results. We do not report the between-group behavioural reaction time results since most of the studies reviewed reported a significant between-group difference in reaction time response, with older subjects consistently responding slower than young subjects (Grady et al., 1998, 2002; Madden et al., 1999; Grossman et al., 2002; Cabeza et al., 2004). There were only two exceptions to this general finding (Daselaar et al., 2003; Morcom et al., 2003). In these two studies there were no significant between-group differences in reaction time.
Reviewed studies
| No. . | Study . | Neuroimaging method . | Cognitive task . | Behavioural performance . |
|---|---|---|---|---|
| 1 | Anderson et al. (2000) | PET | EM encoding and retrieval of word pairs under full attention and divided attention. Retrieval phase involved a forced-choice recognition task.* | Y > O |
| 2 | Cabeza et al. (2004) | fMRI | Verbal WM delayed-response task, verbal EM recognition task and visual attention task.** | Y = O*** |
| 3 | Cabeza et al. (2002)BB | PET | EM retrieval of word pairs. Retrieval phase involved cued recall and source retrieval. | Y > O |
| 4 | Cabeza et al. (2000) | PET | EM retrieval of words. Retrieval phase involved both recognition and temporal order memory tasks. | Y > O |
| 5 | Cabeza et al. (1997)BB | PET | EM encoding and retrieval of word pairs. Retrieval phase included recognition tasks and cued recall tasks. | Y = O |
| 6 | Daselaar et al. (2003)BB | fMRI | EM encoding and retrieval of words. Retrieval phase involved forced-choice recognition. | Y = O |
| 7 | Grady et al. (1999) | PET | EM encoding of words and pictures. Levels of processing manipulation incorporated into encoding phase. | Y = O, encoding; Y > O, post-scan recognition |
| 8 | Grady et al. (1998)BB | PET | WM delayed match-to-sample task for faces. Delay manipulation incorporated: long versus short delay. | Y > O |
| 9 | Grady et al. (2002)BB | PET | EM encoding and retrieval for faces. Levels of processing manipulation during encoding. Retrieval phase involved forced-choice recognition tasks. | Y = O encoding, recognition hits; Y > O, recognition false alarms |
| 10 | Grossman et al. (2002) | fMRI | Sentence comprehension task. WM manipulation involved long versus short antecedent noun-gap linkage. | Y = O |
| 11 | Iidaka et al. (2001)BB | fMRI | Intentional EM encoding of pairs of abstract pictures. | Y > O |
| 12 | Jonides et al. (2000) | PET | Verbal WM item recognition task. | Y = O, overall; Y < O, interference effect |
| 13 | Logan et al. (2002) | fMRI | Experiment 1: EM encoding for faces and words. Experiment 2: levels of processing manipulation incorporated to encoding phase. | Experiment 1: Y > O, post-scan recognition test. Experiment 2: Y = O |
| 14 | Madden et al. (1999)BB | PET | EM encoding and retrieval of words. Retrieval phase involved forced-choice recognition. | Y = O, encoding; Y > O recognition |
| 15 | Mitchell et al. (2000) | fMRI | WM delayed match-to-sample for object, location and combination trials. | Y > O |
| 16 | Morcom et al. (2003)BB | fMRI | EM encoding for words using animacy judgements | Y = O, encoding; Y > O, post-scan recognition |
| 17 | Park et al. (2003) | fMRI | WM delayed-response task for real world scenes. Examined activity differences between a WM maintenance condition and an extended visual condition. | Y = O |
| 18 | Reuer-Lorenz et al. (2000) | PET | WM delayed match-to-sample task for spatial and for verbal information. | Y = O |
| 19 | Rosen et al. (2002)BB | fMRI | EM encoding for words. Levels of processing manipulation at encoding. | Y = O, encoding; Y > O, post-scan recognition |
| 20 | Rypma and D'Esposito (2000) BB | fMRI | WM delayed-response task. Incorporated a WM load manipulation: high versus low load. | Y = O |
| 21 | Schiavetto et al. (2002) | PET | Perceptual matching baselines for object identity and object location. EM encoding and retrieval of objects. Retrieval phase involved forced choice recognition task for either object identity or location. | Y > O |
| 22 | Smith et al. (2001) | PET | Single verbal WM delayed-response task and dual task verbal WM with mathematical operation span. | Y = O |
| No. . | Study . | Neuroimaging method . | Cognitive task . | Behavioural performance . |
|---|---|---|---|---|
| 1 | Anderson et al. (2000) | PET | EM encoding and retrieval of word pairs under full attention and divided attention. Retrieval phase involved a forced-choice recognition task.* | Y > O |
| 2 | Cabeza et al. (2004) | fMRI | Verbal WM delayed-response task, verbal EM recognition task and visual attention task.** | Y = O*** |
| 3 | Cabeza et al. (2002)BB | PET | EM retrieval of word pairs. Retrieval phase involved cued recall and source retrieval. | Y > O |
| 4 | Cabeza et al. (2000) | PET | EM retrieval of words. Retrieval phase involved both recognition and temporal order memory tasks. | Y > O |
| 5 | Cabeza et al. (1997)BB | PET | EM encoding and retrieval of word pairs. Retrieval phase included recognition tasks and cued recall tasks. | Y = O |
| 6 | Daselaar et al. (2003)BB | fMRI | EM encoding and retrieval of words. Retrieval phase involved forced-choice recognition. | Y = O |
| 7 | Grady et al. (1999) | PET | EM encoding of words and pictures. Levels of processing manipulation incorporated into encoding phase. | Y = O, encoding; Y > O, post-scan recognition |
| 8 | Grady et al. (1998)BB | PET | WM delayed match-to-sample task for faces. Delay manipulation incorporated: long versus short delay. | Y > O |
| 9 | Grady et al. (2002)BB | PET | EM encoding and retrieval for faces. Levels of processing manipulation during encoding. Retrieval phase involved forced-choice recognition tasks. | Y = O encoding, recognition hits; Y > O, recognition false alarms |
| 10 | Grossman et al. (2002) | fMRI | Sentence comprehension task. WM manipulation involved long versus short antecedent noun-gap linkage. | Y = O |
| 11 | Iidaka et al. (2001)BB | fMRI | Intentional EM encoding of pairs of abstract pictures. | Y > O |
| 12 | Jonides et al. (2000) | PET | Verbal WM item recognition task. | Y = O, overall; Y < O, interference effect |
| 13 | Logan et al. (2002) | fMRI | Experiment 1: EM encoding for faces and words. Experiment 2: levels of processing manipulation incorporated to encoding phase. | Experiment 1: Y > O, post-scan recognition test. Experiment 2: Y = O |
| 14 | Madden et al. (1999)BB | PET | EM encoding and retrieval of words. Retrieval phase involved forced-choice recognition. | Y = O, encoding; Y > O recognition |
| 15 | Mitchell et al. (2000) | fMRI | WM delayed match-to-sample for object, location and combination trials. | Y > O |
| 16 | Morcom et al. (2003)BB | fMRI | EM encoding for words using animacy judgements | Y = O, encoding; Y > O, post-scan recognition |
| 17 | Park et al. (2003) | fMRI | WM delayed-response task for real world scenes. Examined activity differences between a WM maintenance condition and an extended visual condition. | Y = O |
| 18 | Reuer-Lorenz et al. (2000) | PET | WM delayed match-to-sample task for spatial and for verbal information. | Y = O |
| 19 | Rosen et al. (2002)BB | fMRI | EM encoding for words. Levels of processing manipulation at encoding. | Y = O, encoding; Y > O, post-scan recognition |
| 20 | Rypma and D'Esposito (2000) BB | fMRI | WM delayed-response task. Incorporated a WM load manipulation: high versus low load. | Y = O |
| 21 | Schiavetto et al. (2002) | PET | Perceptual matching baselines for object identity and object location. EM encoding and retrieval of objects. Retrieval phase involved forced choice recognition task for either object identity or location. | Y > O |
| 22 | Smith et al. (2001) | PET | Single verbal WM delayed-response task and dual task verbal WM with mathematical operation span. | Y = O |
List of studies included in the review. The neuro-imaging method column describes whether PET or fMRI was used in the study. The ‘Cognitive task’ column gives a brief description of the tasks tested. The ‘Behaviour performance’ column lists whether there was a significant between group difference in which the elderly performed poorer than the young (Y > O) or whether there were no significant between group differences in accuracy across tasks (Y = O). If a study produced both significant and non-significant effects it is noted for which tasks there was an effect (e.g. ‘Y > O, recognition') and for which tasks there was no effect (e.g. ‘Y=O, encoding'). EM, episodic memory; WM, working memory.
Only the results from full attention conditions were included in this review.
Only the results from the WM and EM tasks are included in this review.
Older subjects had fewer remember responses and more know responses compared to young subjects. BB, highlights papers that conducted either an indirect or a direct examination of brain-behaviour relationships.
Reviewed studies
| No. . | Study . | Neuroimaging method . | Cognitive task . | Behavioural performance . |
|---|---|---|---|---|
| 1 | Anderson et al. (2000) | PET | EM encoding and retrieval of word pairs under full attention and divided attention. Retrieval phase involved a forced-choice recognition task.* | Y > O |
| 2 | Cabeza et al. (2004) | fMRI | Verbal WM delayed-response task, verbal EM recognition task and visual attention task.** | Y = O*** |
| 3 | Cabeza et al. (2002)BB | PET | EM retrieval of word pairs. Retrieval phase involved cued recall and source retrieval. | Y > O |
| 4 | Cabeza et al. (2000) | PET | EM retrieval of words. Retrieval phase involved both recognition and temporal order memory tasks. | Y > O |
| 5 | Cabeza et al. (1997)BB | PET | EM encoding and retrieval of word pairs. Retrieval phase included recognition tasks and cued recall tasks. | Y = O |
| 6 | Daselaar et al. (2003)BB | fMRI | EM encoding and retrieval of words. Retrieval phase involved forced-choice recognition. | Y = O |
| 7 | Grady et al. (1999) | PET | EM encoding of words and pictures. Levels of processing manipulation incorporated into encoding phase. | Y = O, encoding; Y > O, post-scan recognition |
| 8 | Grady et al. (1998)BB | PET | WM delayed match-to-sample task for faces. Delay manipulation incorporated: long versus short delay. | Y > O |
| 9 | Grady et al. (2002)BB | PET | EM encoding and retrieval for faces. Levels of processing manipulation during encoding. Retrieval phase involved forced-choice recognition tasks. | Y = O encoding, recognition hits; Y > O, recognition false alarms |
| 10 | Grossman et al. (2002) | fMRI | Sentence comprehension task. WM manipulation involved long versus short antecedent noun-gap linkage. | Y = O |
| 11 | Iidaka et al. (2001)BB | fMRI | Intentional EM encoding of pairs of abstract pictures. | Y > O |
| 12 | Jonides et al. (2000) | PET | Verbal WM item recognition task. | Y = O, overall; Y < O, interference effect |
| 13 | Logan et al. (2002) | fMRI | Experiment 1: EM encoding for faces and words. Experiment 2: levels of processing manipulation incorporated to encoding phase. | Experiment 1: Y > O, post-scan recognition test. Experiment 2: Y = O |
| 14 | Madden et al. (1999)BB | PET | EM encoding and retrieval of words. Retrieval phase involved forced-choice recognition. | Y = O, encoding; Y > O recognition |
| 15 | Mitchell et al. (2000) | fMRI | WM delayed match-to-sample for object, location and combination trials. | Y > O |
| 16 | Morcom et al. (2003)BB | fMRI | EM encoding for words using animacy judgements | Y = O, encoding; Y > O, post-scan recognition |
| 17 | Park et al. (2003) | fMRI | WM delayed-response task for real world scenes. Examined activity differences between a WM maintenance condition and an extended visual condition. | Y = O |
| 18 | Reuer-Lorenz et al. (2000) | PET | WM delayed match-to-sample task for spatial and for verbal information. | Y = O |
| 19 | Rosen et al. (2002)BB | fMRI | EM encoding for words. Levels of processing manipulation at encoding. | Y = O, encoding; Y > O, post-scan recognition |
| 20 | Rypma and D'Esposito (2000) BB | fMRI | WM delayed-response task. Incorporated a WM load manipulation: high versus low load. | Y = O |
| 21 | Schiavetto et al. (2002) | PET | Perceptual matching baselines for object identity and object location. EM encoding and retrieval of objects. Retrieval phase involved forced choice recognition task for either object identity or location. | Y > O |
| 22 | Smith et al. (2001) | PET | Single verbal WM delayed-response task and dual task verbal WM with mathematical operation span. | Y = O |
| No. . | Study . | Neuroimaging method . | Cognitive task . | Behavioural performance . |
|---|---|---|---|---|
| 1 | Anderson et al. (2000) | PET | EM encoding and retrieval of word pairs under full attention and divided attention. Retrieval phase involved a forced-choice recognition task.* | Y > O |
| 2 | Cabeza et al. (2004) | fMRI | Verbal WM delayed-response task, verbal EM recognition task and visual attention task.** | Y = O*** |
| 3 | Cabeza et al. (2002)BB | PET | EM retrieval of word pairs. Retrieval phase involved cued recall and source retrieval. | Y > O |
| 4 | Cabeza et al. (2000) | PET | EM retrieval of words. Retrieval phase involved both recognition and temporal order memory tasks. | Y > O |
| 5 | Cabeza et al. (1997)BB | PET | EM encoding and retrieval of word pairs. Retrieval phase included recognition tasks and cued recall tasks. | Y = O |
| 6 | Daselaar et al. (2003)BB | fMRI | EM encoding and retrieval of words. Retrieval phase involved forced-choice recognition. | Y = O |
| 7 | Grady et al. (1999) | PET | EM encoding of words and pictures. Levels of processing manipulation incorporated into encoding phase. | Y = O, encoding; Y > O, post-scan recognition |
| 8 | Grady et al. (1998)BB | PET | WM delayed match-to-sample task for faces. Delay manipulation incorporated: long versus short delay. | Y > O |
| 9 | Grady et al. (2002)BB | PET | EM encoding and retrieval for faces. Levels of processing manipulation during encoding. Retrieval phase involved forced-choice recognition tasks. | Y = O encoding, recognition hits; Y > O, recognition false alarms |
| 10 | Grossman et al. (2002) | fMRI | Sentence comprehension task. WM manipulation involved long versus short antecedent noun-gap linkage. | Y = O |
| 11 | Iidaka et al. (2001)BB | fMRI | Intentional EM encoding of pairs of abstract pictures. | Y > O |
| 12 | Jonides et al. (2000) | PET | Verbal WM item recognition task. | Y = O, overall; Y < O, interference effect |
| 13 | Logan et al. (2002) | fMRI | Experiment 1: EM encoding for faces and words. Experiment 2: levels of processing manipulation incorporated to encoding phase. | Experiment 1: Y > O, post-scan recognition test. Experiment 2: Y = O |
| 14 | Madden et al. (1999)BB | PET | EM encoding and retrieval of words. Retrieval phase involved forced-choice recognition. | Y = O, encoding; Y > O recognition |
| 15 | Mitchell et al. (2000) | fMRI | WM delayed match-to-sample for object, location and combination trials. | Y > O |
| 16 | Morcom et al. (2003)BB | fMRI | EM encoding for words using animacy judgements | Y = O, encoding; Y > O, post-scan recognition |
| 17 | Park et al. (2003) | fMRI | WM delayed-response task for real world scenes. Examined activity differences between a WM maintenance condition and an extended visual condition. | Y = O |
| 18 | Reuer-Lorenz et al. (2000) | PET | WM delayed match-to-sample task for spatial and for verbal information. | Y = O |
| 19 | Rosen et al. (2002)BB | fMRI | EM encoding for words. Levels of processing manipulation at encoding. | Y = O, encoding; Y > O, post-scan recognition |
| 20 | Rypma and D'Esposito (2000) BB | fMRI | WM delayed-response task. Incorporated a WM load manipulation: high versus low load. | Y = O |
| 21 | Schiavetto et al. (2002) | PET | Perceptual matching baselines for object identity and object location. EM encoding and retrieval of objects. Retrieval phase involved forced choice recognition task for either object identity or location. | Y > O |
| 22 | Smith et al. (2001) | PET | Single verbal WM delayed-response task and dual task verbal WM with mathematical operation span. | Y = O |
List of studies included in the review. The neuro-imaging method column describes whether PET or fMRI was used in the study. The ‘Cognitive task’ column gives a brief description of the tasks tested. The ‘Behaviour performance’ column lists whether there was a significant between group difference in which the elderly performed poorer than the young (Y > O) or whether there were no significant between group differences in accuracy across tasks (Y = O). If a study produced both significant and non-significant effects it is noted for which tasks there was an effect (e.g. ‘Y > O, recognition') and for which tasks there was no effect (e.g. ‘Y=O, encoding'). EM, episodic memory; WM, working memory.
Only the results from full attention conditions were included in this review.
Only the results from the WM and EM tasks are included in this review.
Older subjects had fewer remember responses and more know responses compared to young subjects. BB, highlights papers that conducted either an indirect or a direct examination of brain-behaviour relationships.
Defining PFC activation
This review focuses on activations reported in the ventral PFC [Brodmann area (BA) 44, 45 and 47 on inferior frontal gyrus (IFG)] dorsal PFC [BA 9 and 46 on middle frontal gyrus (MFG)], and anterior PFC [BA 9 or 10 on superior frontal gyrus (SFG)], since activations in these PFC subregions have been observed in previous neuroimaging studies of WM and EM in young subjects (Fletcher et al., 1997; D'Esposito et al., 1998, 1999b; Henson et al., 1999; Cabeza and Nyberg, 2000; Habib et al., 2003; Nyberg et al., 2003; Wager and Smith, 2003). Figure 1 presents an anatomical image of the human brain, highlighting the regional ventral, dorsal and anterior subdivisions, used in tabulating activations for this review. For each study, results from fMRI or PET activation analyses that directly compared memory tasks to a control task or to one another, were examined. Only task effects yielding PFC activations in young subjects, old subjects, or both, were included in the review. PFC activations reported from higher order interaction effects that were specific to a particular study design, and therefore would not be comparable to result from other studies, were excluded from the review.
Ventral, dorsal and anterior PFC activations reported for young and older subjects from the reviewed WM studies, EM studies of encoding and EM studies of retrieval were tabulated separately by region, respectively (Tables 2–4). Each table was organized into the following columns: the study reviewed, the contrast tested, and the hemispheric location of each activation for each age group, respectively. For studies that employed multivariate partial least squares analysis, we listed the interpretation given for the latent variable results under the ‘contrast tested’ column of the tables. Moreover, we only report main effects from multivariate analyses that were unambiguous: significant differences between tasks and significant age-by-task interactions.
Summary of ventral PFC activations reported across studies
| Study . | Analytical methods . | Contrast . | Hemisphere . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Left . | . | Right . | . | ||||||
. | . | . | Young . | Old . | Young . | Old . | ||||||
| Working memory studies | ||||||||||||
| Cabeza et al. (2004) | Conjunction analysis | WM > baseline | Y | O | Y | O | ||||||
| Grady et al. (1998) | Within-group univariate | WM > control | YY | O | YY | O | ||||||
| Between-group multivariate | Long > short delay | YY | ||||||||||
| Grossman et al. (2002) | Between-group univariate | Long > short linkage | Y | O | YY | |||||||
| Jonides et al. (2000) | Between-group ROI analysis | High > low interference | YY | O | ||||||||
| Mitchell et al. (2000) | Between-group ANOVA | Object WM main effect | Y | O | ||||||||
| Park et al. (2003) | Between-group ROI analysis | WM retrieval > baseline | Y | OO | Y | OO | ||||||
| Reuter-Lorenz et al. (2000) | Between-group VOI analysis | Verbal WM | Y | O | OO | |||||||
| Between-group VOI analysis | Spatial WM | Y | O | |||||||||
| Within-group univariate | WM encoding > fixation | Y | O | Y | O | |||||||
| Within-group univariate | WM maintenance > fixation | Y | O | |||||||||
| Rypma and D'Esposito (2000) | Across group univariate | WM retrieval > fixation | Y | O | Y | O | ||||||
| Smith et al. (2001) | Within-group univariate | Verbal WM > control | YY | |||||||||
| Within-group univariate | Dual-task > single tasks | OO | ||||||||||
| Episodic memory encoding studies | ||||||||||||
| Anderson et al. (2000) | Between-group multivariate PLS | Encoding > retrieval | YY | YY | ||||||||
| Cabeza et al. (1997) | Between-group multivariate PLS | Encoding > retrieval | YY | |||||||||
| Daselaar et al. (2003) | Within-group univariate SPM | Encoding > baseline | Y | O | ||||||||
| Grady et al. (1999) | Between-group multivariate PLS | Levels of processing effect | YY | O | ||||||||
| Grady et al. (2002) | Between-group multivariate PLS | Encoding > control | YY | O | YY | O | ||||||
| Iidaka et al. (2001) | Within-group univariate | Encoding concrete unrelated words > control | Y | O | ||||||||
| Within-group univariate | Encoding abstract pictures > control | Y | O | |||||||||
| Logan et al. (2002) | Between-group ROI analysis | Word encoding > baseline | YY | O | Y | OO | ||||||
| Between-group ROI analysis | Face encoding > baseline | Y | OO | YY | O | |||||||
| Madden et al. (1999) | Within-group univariate | Encoding > baseline | OO | |||||||||
| Morcom et al. (2003) | Between-group univariate | Encoding > baseline related to subsequent memory | Y | O | Y | O | ||||||
| Rosen et al. (2002) | Within-group univariate | Deep > shallow encoding | Y | O | Y | OO | ||||||
| Between-group ROI analysis | Deep > shallow encoding | OO | ||||||||||
| Schiavetto et al. (2002) | Between-group ANOVA | Encoding > retrieval main effect and interaction | OO | |||||||||
| Episodic memory retrieval studies | ||||||||||||
| Anderson et al. (2000) | Between-group multivariate PLS | Retrieval > encoding | OO | Y | O | |||||||
| Cabeza et al. (2004) | Conjunction analysis | Retrieval > baseline | Y | O | Y | O | ||||||
| Cabeza et al. (2002) | Within-group univariate | Cued recall > source memory | Y | OO | ||||||||
| Cabeza et al. (1997) | Between-group multivariate PLS | Retrieval > encoding | OO | YY | O | |||||||
| Daselaar et al. (2003) | Within-group univariate SPM | Retrieval > baseline | Y | O | Y | O | ||||||
| Grady et al. (2002) | Between-group multivariate PLS | Retrieval > encoding | Y | OO | ||||||||
| Madden et al. (1999) | Within-group univariate | Retrieval > baseline | OO | |||||||||
| Schiavetto et al. (2002) | Between-group ANOVA | Retrieval > encoding main effect and interaction | Y | O | ||||||||
| Total sum of reported age-related increases | 11 | 16 | 10 | 7 | ||||||||
| Study . | Analytical methods . | Contrast . | Hemisphere . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Left . | . | Right . | . | ||||||
. | . | . | Young . | Old . | Young . | Old . | ||||||
| Working memory studies | ||||||||||||
| Cabeza et al. (2004) | Conjunction analysis | WM > baseline | Y | O | Y | O | ||||||
| Grady et al. (1998) | Within-group univariate | WM > control | YY | O | YY | O | ||||||
| Between-group multivariate | Long > short delay | YY | ||||||||||
| Grossman et al. (2002) | Between-group univariate | Long > short linkage | Y | O | YY | |||||||
| Jonides et al. (2000) | Between-group ROI analysis | High > low interference | YY | O | ||||||||
| Mitchell et al. (2000) | Between-group ANOVA | Object WM main effect | Y | O | ||||||||
| Park et al. (2003) | Between-group ROI analysis | WM retrieval > baseline | Y | OO | Y | OO | ||||||
| Reuter-Lorenz et al. (2000) | Between-group VOI analysis | Verbal WM | Y | O | OO | |||||||
| Between-group VOI analysis | Spatial WM | Y | O | |||||||||
| Within-group univariate | WM encoding > fixation | Y | O | Y | O | |||||||
| Within-group univariate | WM maintenance > fixation | Y | O | |||||||||
| Rypma and D'Esposito (2000) | Across group univariate | WM retrieval > fixation | Y | O | Y | O | ||||||
| Smith et al. (2001) | Within-group univariate | Verbal WM > control | YY | |||||||||
| Within-group univariate | Dual-task > single tasks | OO | ||||||||||
| Episodic memory encoding studies | ||||||||||||
| Anderson et al. (2000) | Between-group multivariate PLS | Encoding > retrieval | YY | YY | ||||||||
| Cabeza et al. (1997) | Between-group multivariate PLS | Encoding > retrieval | YY | |||||||||
| Daselaar et al. (2003) | Within-group univariate SPM | Encoding > baseline | Y | O | ||||||||
| Grady et al. (1999) | Between-group multivariate PLS | Levels of processing effect | YY | O | ||||||||
| Grady et al. (2002) | Between-group multivariate PLS | Encoding > control | YY | O | YY | O | ||||||
| Iidaka et al. (2001) | Within-group univariate | Encoding concrete unrelated words > control | Y | O | ||||||||
| Within-group univariate | Encoding abstract pictures > control | Y | O | |||||||||
| Logan et al. (2002) | Between-group ROI analysis | Word encoding > baseline | YY | O | Y | OO | ||||||
| Between-group ROI analysis | Face encoding > baseline | Y | OO | YY | O | |||||||
| Madden et al. (1999) | Within-group univariate | Encoding > baseline | OO | |||||||||
| Morcom et al. (2003) | Between-group univariate | Encoding > baseline related to subsequent memory | Y | O | Y | O | ||||||
| Rosen et al. (2002) | Within-group univariate | Deep > shallow encoding | Y | O | Y | OO | ||||||
| Between-group ROI analysis | Deep > shallow encoding | OO | ||||||||||
| Schiavetto et al. (2002) | Between-group ANOVA | Encoding > retrieval main effect and interaction | OO | |||||||||
| Episodic memory retrieval studies | ||||||||||||
| Anderson et al. (2000) | Between-group multivariate PLS | Retrieval > encoding | OO | Y | O | |||||||
| Cabeza et al. (2004) | Conjunction analysis | Retrieval > baseline | Y | O | Y | O | ||||||
| Cabeza et al. (2002) | Within-group univariate | Cued recall > source memory | Y | OO | ||||||||
| Cabeza et al. (1997) | Between-group multivariate PLS | Retrieval > encoding | OO | YY | O | |||||||
| Daselaar et al. (2003) | Within-group univariate SPM | Retrieval > baseline | Y | O | Y | O | ||||||
| Grady et al. (2002) | Between-group multivariate PLS | Retrieval > encoding | Y | OO | ||||||||
| Madden et al. (1999) | Within-group univariate | Retrieval > baseline | OO | |||||||||
| Schiavetto et al. (2002) | Between-group ANOVA | Retrieval > encoding main effect and interaction | Y | O | ||||||||
| Total sum of reported age-related increases | 11 | 16 | 10 | 7 | ||||||||
This table presents reviewed studies that reported ventral PFC activity. Reports of ventral PFC activity are listed first by study type (first column; WM, for working memory and EM, for episodic memory). The method of neuroimaging analysis and contrast effects yielding ventral PFC activations are presented in columns two and three, respectively. The activations are organized by cerebral hemisphere (Left versus Right) and by age group (Young versus Old). Y's and O's are used to notate the activations reported in Young and Old subject groups across studies, respectively. Y, ventral PFC activity in young subjects; O, ventral PFC activity in old subjects; YY, the authors reported young only or young greater than old activity (Y > O) activity in this region for a specific task effect. The bottom row of the table represents the total weighted sum in this region for a specific task effect. OO, the authors reported old only or old greater than young (O > Y) of reported left/right ventral PFC activation in young or old subjects across the studies presented (refer to the Methods section to see how the sum was calculated).
Summary of ventral PFC activations reported across studies
| Study . | Analytical methods . | Contrast . | Hemisphere . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Left . | . | Right . | . | ||||||
. | . | . | Young . | Old . | Young . | Old . | ||||||
| Working memory studies | ||||||||||||
| Cabeza et al. (2004) | Conjunction analysis | WM > baseline | Y | O | Y | O | ||||||
| Grady et al. (1998) | Within-group univariate | WM > control | YY | O | YY | O | ||||||
| Between-group multivariate | Long > short delay | YY | ||||||||||
| Grossman et al. (2002) | Between-group univariate | Long > short linkage | Y | O | YY | |||||||
| Jonides et al. (2000) | Between-group ROI analysis | High > low interference | YY | O | ||||||||
| Mitchell et al. (2000) | Between-group ANOVA | Object WM main effect | Y | O | ||||||||
| Park et al. (2003) | Between-group ROI analysis | WM retrieval > baseline | Y | OO | Y | OO | ||||||
| Reuter-Lorenz et al. (2000) | Between-group VOI analysis | Verbal WM | Y | O | OO | |||||||
| Between-group VOI analysis | Spatial WM | Y | O | |||||||||
| Within-group univariate | WM encoding > fixation | Y | O | Y | O | |||||||
| Within-group univariate | WM maintenance > fixation | Y | O | |||||||||
| Rypma and D'Esposito (2000) | Across group univariate | WM retrieval > fixation | Y | O | Y | O | ||||||
| Smith et al. (2001) | Within-group univariate | Verbal WM > control | YY | |||||||||
| Within-group univariate | Dual-task > single tasks | OO | ||||||||||
| Episodic memory encoding studies | ||||||||||||
| Anderson et al. (2000) | Between-group multivariate PLS | Encoding > retrieval | YY | YY | ||||||||
| Cabeza et al. (1997) | Between-group multivariate PLS | Encoding > retrieval | YY | |||||||||
| Daselaar et al. (2003) | Within-group univariate SPM | Encoding > baseline | Y | O | ||||||||
| Grady et al. (1999) | Between-group multivariate PLS | Levels of processing effect | YY | O | ||||||||
| Grady et al. (2002) | Between-group multivariate PLS | Encoding > control | YY | O | YY | O | ||||||
| Iidaka et al. (2001) | Within-group univariate | Encoding concrete unrelated words > control | Y | O | ||||||||
| Within-group univariate | Encoding abstract pictures > control | Y | O | |||||||||
| Logan et al. (2002) | Between-group ROI analysis | Word encoding > baseline | YY | O | Y | OO | ||||||
| Between-group ROI analysis | Face encoding > baseline | Y | OO | YY | O | |||||||
| Madden et al. (1999) | Within-group univariate | Encoding > baseline | OO | |||||||||
| Morcom et al. (2003) | Between-group univariate | Encoding > baseline related to subsequent memory | Y | O | Y | O | ||||||
| Rosen et al. (2002) | Within-group univariate | Deep > shallow encoding | Y | O | Y | OO | ||||||
| Between-group ROI analysis | Deep > shallow encoding | OO | ||||||||||
| Schiavetto et al. (2002) | Between-group ANOVA | Encoding > retrieval main effect and interaction | OO | |||||||||
| Episodic memory retrieval studies | ||||||||||||
| Anderson et al. (2000) | Between-group multivariate PLS | Retrieval > encoding | OO | Y | O | |||||||
| Cabeza et al. (2004) | Conjunction analysis | Retrieval > baseline | Y | O | Y | O | ||||||
| Cabeza et al. (2002) | Within-group univariate | Cued recall > source memory | Y | OO | ||||||||
| Cabeza et al. (1997) | Between-group multivariate PLS | Retrieval > encoding | OO | YY | O | |||||||
| Daselaar et al. (2003) | Within-group univariate SPM | Retrieval > baseline | Y | O | Y | O | ||||||
| Grady et al. (2002) | Between-group multivariate PLS | Retrieval > encoding | Y | OO | ||||||||
| Madden et al. (1999) | Within-group univariate | Retrieval > baseline | OO | |||||||||
| Schiavetto et al. (2002) | Between-group ANOVA | Retrieval > encoding main effect and interaction | Y | O | ||||||||
| Total sum of reported age-related increases | 11 | 16 | 10 | 7 | ||||||||
| Study . | Analytical methods . | Contrast . | Hemisphere . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Left . | . | Right . | . | ||||||
. | . | . | Young . | Old . | Young . | Old . | ||||||
| Working memory studies | ||||||||||||
| Cabeza et al. (2004) | Conjunction analysis | WM > baseline | Y | O | Y | O | ||||||
| Grady et al. (1998) | Within-group univariate | WM > control | YY | O | YY | O | ||||||
| Between-group multivariate | Long > short delay | YY | ||||||||||
| Grossman et al. (2002) | Between-group univariate | Long > short linkage | Y | O | YY | |||||||
| Jonides et al. (2000) | Between-group ROI analysis | High > low interference | YY | O | ||||||||
| Mitchell et al. (2000) | Between-group ANOVA | Object WM main effect | Y | O | ||||||||
| Park et al. (2003) | Between-group ROI analysis | WM retrieval > baseline | Y | OO | Y | OO | ||||||
| Reuter-Lorenz et al. (2000) | Between-group VOI analysis | Verbal WM | Y | O | OO | |||||||
| Between-group VOI analysis | Spatial WM | Y | O | |||||||||
| Within-group univariate | WM encoding > fixation | Y | O | Y | O | |||||||
| Within-group univariate | WM maintenance > fixation | Y | O | |||||||||
| Rypma and D'Esposito (2000) | Across group univariate | WM retrieval > fixation | Y | O | Y | O | ||||||
| Smith et al. (2001) | Within-group univariate | Verbal WM > control | YY | |||||||||
| Within-group univariate | Dual-task > single tasks | OO | ||||||||||
| Episodic memory encoding studies | ||||||||||||
| Anderson et al. (2000) | Between-group multivariate PLS | Encoding > retrieval | YY | YY | ||||||||
| Cabeza et al. (1997) | Between-group multivariate PLS | Encoding > retrieval | YY | |||||||||
| Daselaar et al. (2003) | Within-group univariate SPM | Encoding > baseline | Y | O | ||||||||
| Grady et al. (1999) | Between-group multivariate PLS | Levels of processing effect | YY | O | ||||||||
| Grady et al. (2002) | Between-group multivariate PLS | Encoding > control | YY | O | YY | O | ||||||
| Iidaka et al. (2001) | Within-group univariate | Encoding concrete unrelated words > control | Y | O | ||||||||
| Within-group univariate | Encoding abstract pictures > control | Y | O | |||||||||
| Logan et al. (2002) | Between-group ROI analysis | Word encoding > baseline | YY | O | Y | OO | ||||||
| Between-group ROI analysis | Face encoding > baseline | Y | OO | YY | O | |||||||
| Madden et al. (1999) | Within-group univariate | Encoding > baseline | OO | |||||||||
| Morcom et al. (2003) | Between-group univariate | Encoding > baseline related to subsequent memory | Y | O | Y | O | ||||||
| Rosen et al. (2002) | Within-group univariate | Deep > shallow encoding | Y | O | Y | OO | ||||||
| Between-group ROI analysis | Deep > shallow encoding | OO | ||||||||||
| Schiavetto et al. (2002) | Between-group ANOVA | Encoding > retrieval main effect and interaction | OO | |||||||||
| Episodic memory retrieval studies | ||||||||||||
| Anderson et al. (2000) | Between-group multivariate PLS | Retrieval > encoding | OO | Y | O | |||||||
| Cabeza et al. (2004) | Conjunction analysis | Retrieval > baseline | Y | O | Y | O | ||||||
| Cabeza et al. (2002) | Within-group univariate | Cued recall > source memory | Y | OO | ||||||||
| Cabeza et al. (1997) | Between-group multivariate PLS | Retrieval > encoding | OO | YY | O | |||||||
| Daselaar et al. (2003) | Within-group univariate SPM | Retrieval > baseline | Y | O | Y | O | ||||||
| Grady et al. (2002) | Between-group multivariate PLS | Retrieval > encoding | Y | OO | ||||||||
| Madden et al. (1999) | Within-group univariate | Retrieval > baseline | OO | |||||||||
| Schiavetto et al. (2002) | Between-group ANOVA | Retrieval > encoding main effect and interaction | Y | O | ||||||||
| Total sum of reported age-related increases | 11 | 16 | 10 | 7 | ||||||||
This table presents reviewed studies that reported ventral PFC activity. Reports of ventral PFC activity are listed first by study type (first column; WM, for working memory and EM, for episodic memory). The method of neuroimaging analysis and contrast effects yielding ventral PFC activations are presented in columns two and three, respectively. The activations are organized by cerebral hemisphere (Left versus Right) and by age group (Young versus Old). Y's and O's are used to notate the activations reported in Young and Old subject groups across studies, respectively. Y, ventral PFC activity in young subjects; O, ventral PFC activity in old subjects; YY, the authors reported young only or young greater than old activity (Y > O) activity in this region for a specific task effect. The bottom row of the table represents the total weighted sum in this region for a specific task effect. OO, the authors reported old only or old greater than young (O > Y) of reported left/right ventral PFC activation in young or old subjects across the studies presented (refer to the Methods section to see how the sum was calculated).
The gyral location attributed to a particular PFC activation was based on the tabulated results reported by the authors of each reviewed study. For papers that did not define the gyral location of PFC activations, but instead reported Brodmann areas (BA) or used a different naming scheme for anatomical regions, we converted the coordinates to Talairach and Tournoux space if they were presented in the Montreal Neurological Institute (MNI) space and determined the gyral location using the Talairach and Tournoux atlas (Talairach and Tournoux, 1988). In addition, for studies which reported BA 10 activity as being localised in MFG (Grady et al., 1999, 2002; Madden et al., 1999; Anderson et al., 2000), each BA 10 coordinate was located using the Tailarach and Tournoux atlas to determine whether these activations were: (i) in MFG but on the cusp of BA 9 or 46 (ii) actually in anterior PFC (in SFG) or in the anterior cingulate or (iii) in IFG on the cusp of BA 44. Based on this re-examination, activations labelled as BA 10 in MFG were re-localized as being in the ventral, dorsal or anterior PFC, accordingly. Group differences in regional activations were based on the interpretations put forth by the authors of each paper, due to either their qualitative comparisons of the within-group results (differences in univariate t-statistic values for brain regions reported) or based on direct between-group comparisons.
We also report a weighted tabulated sum of the age-related differences in activation reported for each region in the left and right hemisphere. We calculated this sum in the following manner: (i) studies in which equivalent level of activation is reported for both age groups were given a weight = 0; (ii) studies in which both groups activated a particular region, but one age group exhibited a greater degree of activity (based on within group t-statistic values and the authors' interpretations of the within group results) were given a weight = 1; and (iii) studies in which activity in a particular region was reported for only one age group were given a weight = 2. These weighted values were then summed up for each age group and each hemisphere. This weighted sum is a summary index to quantify group differences in activation of a particular region across a task domain.
Examination of brain-behaviour relationships
In the Discussion section we draw on the results from brain-behaviour analyses conducted by some of the studies reviewed. These relationships were examined directly and indirectly. Direct statistical examination used correlation, multivariate or multiple regression methods and was conducted in 6 of the 22 papers reviewed (Grady et al., 1998, 2002; Iidaka et al., 2002; Madden et al., 1999; Morcom et al., 2003; Rypma and D'Esposito, 2000). Indirect examinations of brain-behaviour relationships were conducted in three studies reviewed (Cabeza et al., 2002; Rosen et al., 2002; Daselaar et al., 2003). In these studies older subjects were split into two groups based on behavioural performance: high performers, who performed equivalently to young subjects, and low performers, who performed poorer than high performing older and young subjects. Brain activity differences were then examined between these groups.
Results
The results of this review are presented in two sections. First, we summarize the age-related differences in regional PFC activations. Second, we examine hemispheric differences in age-related deficits in PFC function.
Region-specific changes in PFC activity
Ventral PFC
WM studies
The majority of the WM studies reviewed used variations of traditional delay tasks. Different studies focused on different WM operations (encoding, delay, retrieval) or stimulus or load manipulations. However, the number of studies that focused on any single cognitive operation were few. Thus, we do not differentiate between age-related differences in PFC activity within a particular WM operation or manipulation in this review.
In general, both young and elderly show similar patterns of PFC activation across WM studies. In a few studies, older subjects exhibited less left ventral PFC activity [3/9 studies; Grady et al., 1998; Jonides et al., 2000; Smith et al., 2001] and less right ventral PFC activity [2/9 studies; Grady et al., 1998; Grossman et al., 2002] compared to young subjects. In three studies ventral PFC was over-recruited in older subjects (Reuter-Lorenz et al., 2000; Smith et al., 2001; Park et al., 2003).
EM encoding studies
Both age groups exhibit left-biased ventral PFC activity during encoding but older subjects exhibited less left ventral PFC activity compared to young subjects (6/12 studies; see Table 2). In addition, in three of these studies the elderly also exhibited less right ventral PFC activity compared to young subjects (Anderson et al., 2000; Grady et al., 2002; Logan et al., 2002). However, in the Logan et al. (2002) study, the elderly activated the contralateral ventral PFC region to a greater extent during word encoding (right > left in elderly) and face encoding (left > right in elderly), compared to the young subjects.
The elderly over-recruited the ventral PFC in three studies (Madden et al., 1999; Rosen et al., 2002; Schiavetto et al., 2002). In two of these studies this over-recruitment was left lateralized (Madden et al., 1999; Schiavetto et al., 2002) and in the other it was right lateralized (Rosen et al., 2002). Overall, the studies show that both age groups activate the bilateral ventral PFC during EM encoding; however, there is greater activity in the left ventral PFC compared to the right ventral PFC and young subjects activate the left ventral PFC to a greater degree than older subjects.
EM retrieval studies
Older subjects consistently exhibit greater left ventral PFC during retrieval as compared to young subjects (6/8 studies; see Table 2). In contrast, Cabeza et al. (1997) report greater right ventral PFC activity in young versus older subjects.
Summary
Across task domains both age groups activate the ventral PFC. More specifically, during WM and EM encoding, older subjects do not the engage ventral PFC to the same degree as young subjects. In contrast, during EM retrieval, older subjects over-recruit the ventral PFC, especially in the left hemisphere. Combined, these results suggest reduced task-specificity in ventral PFC function with age.
Dorsal PFC
WM studies
Older subjects exhibited greater dorsal PFC activity, especially in the left hemisphere, compared to young subjects (5/7 studies; see Table 3).
Summary of dorsal PFC activations reported across studies
| Study . | Analytical methods . | Contrast . | Hemisphere . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Left . | . | Right . | . | ||||||
. | . | . | Young . | Old . | Young . | Old . | ||||||
| Working memory studies | ||||||||||||
| Cabeza et al. (2004) | Conjunction analysis | WM > baseline | Y | O | ||||||||
| Between-group univariate analysis | WM > baseline | OO | OO | |||||||||
| Grady et al. (1998) | Within-group univariate | WM > control | OO | |||||||||
| Between-group multivariate | Long > short delay | Y | OO | |||||||||
| Grossman et al. (2002) | Between-group univariate | Long > short linkage | OO | |||||||||
| Mitchell et al. (2000) | Between-group ANOVA | Object WM | Y | O | ||||||||
| Reuter-Lorenz et al. (2000) | Between-group VOI analysis | Verbal WM | YY | OO | ||||||||
| Between-group VOI analysis | Spatial WM | OO | YY | |||||||||
| Within-group univariate | WM encoding > fixation | Y | O | |||||||||
| Within-group univariate | WM maintenance > fixation | Y | O | |||||||||
| Rypma and D'Esposito (2000)BB | Across group univariate | WM retrieval > fixation | Y | O | ||||||||
| Within-group ROI analysis | WM retrieval: High > low load | YY | O | |||||||||
| Smith et al. (2001) | Within-group ROI analysis | Verbal WM > control | Y | O | YY | |||||||
| Within-group ROI analysis | Dual-task > single tasks | OO | ||||||||||
| Episodic memory encoding studies | ||||||||||||
| Anderson et al. (2000) | Between-group multivariate PLS | Encoding > retrieval | Y | O | OO | |||||||
| Cabeza et al. (1997) | Between-group multivariate PLS | Encoding > retrieval | YY | O | ||||||||
| Grady et al. (1999) | Between-group multivariate PLS | Verbal > object encoding | YY | O | YY | O | ||||||
| Between-group multivariate PLS | Intentional > semantic encoding | YY | O | |||||||||
| Between-group multivariate PLS | Levels of processing effect | YY | O | |||||||||
| Grady et al. (2002) | Between-group multivariate PLS | Encoding > control | YY | O | ||||||||
| Iidaka et al. (2001) | Within-group univariate | Encoding concrete unrelated words > control | YY | |||||||||
| Within-group univariate | Encoding abstract pictures > control | YY | ||||||||||
| Madden et al. (1999) | Within-group univariate | Encoding > baseline | OO | |||||||||
| Morcom et al. (2003) | Between-group univariate | Encoding > baseline related to subsequent memory | Y | O | ||||||||
| Rosen et al. (2002) | Within-group univariate | Deep > Shallow Encoding | Y | O | ||||||||
| Episodic memory retrieval studies | ||||||||||||
| Anderson et al. (2000) | Between-group multivariate PLS | Retrieval > encoding | YY | YY | ||||||||
| Cabeza et al. (2004) | Conjunction analysis | Retrieval > baseline | Y | O | Y | O | ||||||
| Cabeza et al. (2002) | Within-group univariate | Cued recall > source memory | YY | |||||||||
| Within-group univariate | Source memory > cued recall | YY | ||||||||||
| Cabeza et al. (2000) | Between-group ANOVA | Temporal order > item retrieval | Y | O | ||||||||
| Cabeza et al. (1997) | Between-group multivariate PLS | Retrieval > encoding | YY | O | ||||||||
| Daselaar et al. (2003) | Within-group univariate SPM | Retrieval > baseline | OO | |||||||||
| Grady et al. (2002) | Between-group multivariate PLS | Retrieval > control | Y | O | YY | |||||||
| Between-group multivariate PLS | Retrieval > encoding | Y | O | Y | OO | |||||||
| Madden et al. (1999) | Between-group univariate | Retrieval > baseline | OO | OO | ||||||||
| Schiavetto et al. (2002) | Between-group ANOVA | Retrieval > encoding main effect and interaction | Y | O | Y | O | ||||||
| Total sum of reported age-related increases | 9 | 15 | 19 | 11 | ||||||||
| Study . | Analytical methods . | Contrast . | Hemisphere . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Left . | . | Right . | . | ||||||
. | . | . | Young . | Old . | Young . | Old . | ||||||
| Working memory studies | ||||||||||||
| Cabeza et al. (2004) | Conjunction analysis | WM > baseline | Y | O | ||||||||
| Between-group univariate analysis | WM > baseline | OO | OO | |||||||||
| Grady et al. (1998) | Within-group univariate | WM > control | OO | |||||||||
| Between-group multivariate | Long > short delay | Y | OO | |||||||||
| Grossman et al. (2002) | Between-group univariate | Long > short linkage | OO | |||||||||
| Mitchell et al. (2000) | Between-group ANOVA | Object WM | Y | O | ||||||||
| Reuter-Lorenz et al. (2000) | Between-group VOI analysis | Verbal WM | YY | OO | ||||||||
| Between-group VOI analysis | Spatial WM | OO | YY | |||||||||
| Within-group univariate | WM encoding > fixation | Y | O | |||||||||
| Within-group univariate | WM maintenance > fixation | Y | O | |||||||||
| Rypma and D'Esposito (2000)BB | Across group univariate | WM retrieval > fixation | Y | O | ||||||||
| Within-group ROI analysis | WM retrieval: High > low load | YY | O | |||||||||
| Smith et al. (2001) | Within-group ROI analysis | Verbal WM > control | Y | O | YY | |||||||
| Within-group ROI analysis | Dual-task > single tasks | OO | ||||||||||
| Episodic memory encoding studies | ||||||||||||
| Anderson et al. (2000) | Between-group multivariate PLS | Encoding > retrieval | Y | O | OO | |||||||
| Cabeza et al. (1997) | Between-group multivariate PLS | Encoding > retrieval | YY | O | ||||||||
| Grady et al. (1999) | Between-group multivariate PLS | Verbal > object encoding | YY | O | YY | O | ||||||
| Between-group multivariate PLS | Intentional > semantic encoding | YY | O | |||||||||
| Between-group multivariate PLS | Levels of processing effect | YY | O | |||||||||
| Grady et al. (2002) | Between-group multivariate PLS | Encoding > control | YY | O | ||||||||
| Iidaka et al. (2001) | Within-group univariate | Encoding concrete unrelated words > control | YY | |||||||||
| Within-group univariate | Encoding abstract pictures > control | YY | ||||||||||
| Madden et al. (1999) | Within-group univariate | Encoding > baseline | OO | |||||||||
| Morcom et al. (2003) | Between-group univariate | Encoding > baseline related to subsequent memory | Y | O | ||||||||
| Rosen et al. (2002) | Within-group univariate | Deep > Shallow Encoding | Y | O | ||||||||
| Episodic memory retrieval studies | ||||||||||||
| Anderson et al. (2000) | Between-group multivariate PLS | Retrieval > encoding | YY | YY | ||||||||
| Cabeza et al. (2004) | Conjunction analysis | Retrieval > baseline | Y | O | Y | O | ||||||
| Cabeza et al. (2002) | Within-group univariate | Cued recall > source memory | YY | |||||||||
| Within-group univariate | Source memory > cued recall | YY | ||||||||||
| Cabeza et al. (2000) | Between-group ANOVA | Temporal order > item retrieval | Y | O | ||||||||
| Cabeza et al. (1997) | Between-group multivariate PLS | Retrieval > encoding | YY | O | ||||||||
| Daselaar et al. (2003) | Within-group univariate SPM | Retrieval > baseline | OO | |||||||||
| Grady et al. (2002) | Between-group multivariate PLS | Retrieval > control | Y | O | YY | |||||||
| Between-group multivariate PLS | Retrieval > encoding | Y | O | Y | OO | |||||||
| Madden et al. (1999) | Between-group univariate | Retrieval > baseline | OO | OO | ||||||||
| Schiavetto et al. (2002) | Between-group ANOVA | Retrieval > encoding main effect and interaction | Y | O | Y | O | ||||||
| Total sum of reported age-related increases | 9 | 15 | 19 | 11 | ||||||||
This table presents reviewed studies that reported dorsal PFC activity. Reports of dorsal PFC activity are listed first by study type (first column; WM, for working memory and EM, for episodic memory). The method of neuroimaging analysis and contrast effects yielding dorsal PFC activations are presented in columns two and three, respectively. The activations are organized by cerebral hemisphere (Left versus Right) and by age group (Young versus Old). Y's and O's are used to notate the activations reported in Young and Old subject groups across studies, respectively. Y, dorsal PFC activity in young subjects; O, dorsal PFC activity in old subjects; YY, the authors reported young only or young greater than old activity (Y > O) in this region for a specific task effect; OO, the authors reported old only or old greater than young (O > Y) activity in this region for a specific task effect. The bottom row of the table represents the total weighted sum of reported left/right dorsal PFC activation in young and old subjects across the studies presented (refer to the Methods section to see how the sum was calculated).
Summary of dorsal PFC activations reported across studies
| Study . | Analytical methods . | Contrast . | Hemisphere . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Left . | . | Right . | . | ||||||
. | . | . | Young . | Old . | Young . | Old . | ||||||
| Working memory studies | ||||||||||||
| Cabeza et al. (2004) | Conjunction analysis | WM > baseline | Y | O | ||||||||
| Between-group univariate analysis | WM > baseline | OO | OO | |||||||||
| Grady et al. (1998) | Within-group univariate | WM > control | OO | |||||||||
| Between-group multivariate | Long > short delay | Y | OO | |||||||||
| Grossman et al. (2002) | Between-group univariate | Long > short linkage | OO | |||||||||
| Mitchell et al. (2000) | Between-group ANOVA | Object WM | Y | O | ||||||||
| Reuter-Lorenz et al. (2000) | Between-group VOI analysis | Verbal WM | YY | OO | ||||||||
| Between-group VOI analysis | Spatial WM | OO | YY | |||||||||
| Within-group univariate | WM encoding > fixation | Y | O | |||||||||
| Within-group univariate | WM maintenance > fixation | Y | O | |||||||||
| Rypma and D'Esposito (2000)BB | Across group univariate | WM retrieval > fixation | Y | O | ||||||||
| Within-group ROI analysis | WM retrieval: High > low load | YY | O | |||||||||
| Smith et al. (2001) | Within-group ROI analysis | Verbal WM > control | Y | O | YY | |||||||
| Within-group ROI analysis | Dual-task > single tasks | OO | ||||||||||
| Episodic memory encoding studies | ||||||||||||
| Anderson et al. (2000) | Between-group multivariate PLS | Encoding > retrieval | Y | O | OO | |||||||
| Cabeza et al. (1997) | Between-group multivariate PLS | Encoding > retrieval | YY | O | ||||||||
| Grady et al. (1999) | Between-group multivariate PLS | Verbal > object encoding | YY | O | YY | O | ||||||
| Between-group multivariate PLS | Intentional > semantic encoding | YY | O | |||||||||
| Between-group multivariate PLS | Levels of processing effect | YY | O | |||||||||
| Grady et al. (2002) | Between-group multivariate PLS | Encoding > control | YY | O | ||||||||
| Iidaka et al. (2001) | Within-group univariate | Encoding concrete unrelated words > control | YY | |||||||||
| Within-group univariate | Encoding abstract pictures > control | YY | ||||||||||
| Madden et al. (1999) | Within-group univariate | Encoding > baseline | OO | |||||||||
| Morcom et al. (2003) | Between-group univariate | Encoding > baseline related to subsequent memory | Y | O | ||||||||
| Rosen et al. (2002) | Within-group univariate | Deep > Shallow Encoding | Y | O | ||||||||
| Episodic memory retrieval studies | ||||||||||||
| Anderson et al. (2000) | Between-group multivariate PLS | Retrieval > encoding | YY | YY | ||||||||
| Cabeza et al. (2004) | Conjunction analysis | Retrieval > baseline | Y | O | Y | O | ||||||
| Cabeza et al. (2002) | Within-group univariate | Cued recall > source memory | YY | |||||||||
| Within-group univariate | Source memory > cued recall | YY | ||||||||||
| Cabeza et al. (2000) | Between-group ANOVA | Temporal order > item retrieval | Y | O | ||||||||
| Cabeza et al. (1997) | Between-group multivariate PLS | Retrieval > encoding | YY | O | ||||||||
| Daselaar et al. (2003) | Within-group univariate SPM | Retrieval > baseline | OO | |||||||||
| Grady et al. (2002) | Between-group multivariate PLS | Retrieval > control | Y | O | YY | |||||||
| Between-group multivariate PLS | Retrieval > encoding | Y | O | Y | OO | |||||||
| Madden et al. (1999) | Between-group univariate | Retrieval > baseline | OO | OO | ||||||||
| Schiavetto et al. (2002) | Between-group ANOVA | Retrieval > encoding main effect and interaction | Y | O | Y | O | ||||||
| Total sum of reported age-related increases | 9 | 15 | 19 | 11 | ||||||||
| Study . | Analytical methods . | Contrast . | Hemisphere . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Left . | . | Right . | . | ||||||
. | . | . | Young . | Old . | Young . | Old . | ||||||
| Working memory studies | ||||||||||||
| Cabeza et al. (2004) | Conjunction analysis | WM > baseline | Y | O | ||||||||
| Between-group univariate analysis | WM > baseline | OO | OO | |||||||||
| Grady et al. (1998) | Within-group univariate | WM > control | OO | |||||||||
| Between-group multivariate | Long > short delay | Y | OO | |||||||||
| Grossman et al. (2002) | Between-group univariate | Long > short linkage | OO | |||||||||
| Mitchell et al. (2000) | Between-group ANOVA | Object WM | Y | O | ||||||||
| Reuter-Lorenz et al. (2000) | Between-group VOI analysis | Verbal WM | YY | OO | ||||||||
| Between-group VOI analysis | Spatial WM | OO | YY | |||||||||
| Within-group univariate | WM encoding > fixation | Y | O | |||||||||
| Within-group univariate | WM maintenance > fixation | Y | O | |||||||||
| Rypma and D'Esposito (2000)BB | Across group univariate | WM retrieval > fixation | Y | O | ||||||||
| Within-group ROI analysis | WM retrieval: High > low load | YY | O | |||||||||
| Smith et al. (2001) | Within-group ROI analysis | Verbal WM > control | Y | O | YY | |||||||
| Within-group ROI analysis | Dual-task > single tasks | OO | ||||||||||
| Episodic memory encoding studies | ||||||||||||
| Anderson et al. (2000) | Between-group multivariate PLS | Encoding > retrieval | Y | O | OO | |||||||
| Cabeza et al. (1997) | Between-group multivariate PLS | Encoding > retrieval | YY | O | ||||||||
| Grady et al. (1999) | Between-group multivariate PLS | Verbal > object encoding | YY | O | YY | O | ||||||
| Between-group multivariate PLS | Intentional > semantic encoding | YY | O | |||||||||
| Between-group multivariate PLS | Levels of processing effect | YY | O | |||||||||
| Grady et al. (2002) | Between-group multivariate PLS | Encoding > control | YY | O | ||||||||
| Iidaka et al. (2001) | Within-group univariate | Encoding concrete unrelated words > control | YY | |||||||||
| Within-group univariate | Encoding abstract pictures > control | YY | ||||||||||
| Madden et al. (1999) | Within-group univariate | Encoding > baseline | OO | |||||||||
| Morcom et al. (2003) | Between-group univariate | Encoding > baseline related to subsequent memory | Y | O | ||||||||
| Rosen et al. (2002) | Within-group univariate | Deep > Shallow Encoding | Y | O | ||||||||
| Episodic memory retrieval studies | ||||||||||||
| Anderson et al. (2000) | Between-group multivariate PLS | Retrieval > encoding | YY | YY | ||||||||
| Cabeza et al. (2004) | Conjunction analysis | Retrieval > baseline | Y | O | Y | O | ||||||
| Cabeza et al. (2002) | Within-group univariate | Cued recall > source memory | YY | |||||||||
| Within-group univariate | Source memory > cued recall | YY | ||||||||||
| Cabeza et al. (2000) | Between-group ANOVA | Temporal order > item retrieval | Y | O | ||||||||
| Cabeza et al. (1997) | Between-group multivariate PLS | Retrieval > encoding | YY | O | ||||||||
| Daselaar et al. (2003) | Within-group univariate SPM | Retrieval > baseline | OO | |||||||||
| Grady et al. (2002) | Between-group multivariate PLS | Retrieval > control | Y | O | YY | |||||||
| Between-group multivariate PLS | Retrieval > encoding | Y | O | Y | OO | |||||||
| Madden et al. (1999) | Between-group univariate | Retrieval > baseline | OO | OO | ||||||||
| Schiavetto et al. (2002) | Between-group ANOVA | Retrieval > encoding main effect and interaction | Y | O | Y | O | ||||||
| Total sum of reported age-related increases | 9 | 15 | 19 | 11 | ||||||||
This table presents reviewed studies that reported dorsal PFC activity. Reports of dorsal PFC activity are listed first by study type (first column; WM, for working memory and EM, for episodic memory). The method of neuroimaging analysis and contrast effects yielding dorsal PFC activations are presented in columns two and three, respectively. The activations are organized by cerebral hemisphere (Left versus Right) and by age group (Young versus Old). Y's and O's are used to notate the activations reported in Young and Old subject groups across studies, respectively. Y, dorsal PFC activity in young subjects; O, dorsal PFC activity in old subjects; YY, the authors reported young only or young greater than old activity (Y > O) in this region for a specific task effect; OO, the authors reported old only or old greater than young (O > Y) activity in this region for a specific task effect. The bottom row of the table represents the total weighted sum of reported left/right dorsal PFC activation in young and old subjects across the studies presented (refer to the Methods section to see how the sum was calculated).
EM encoding studies
Overall, young subjects exhibited greater dorsal PFC activity compared to older subjects (5/9 studies; see Table 3). In two of these studies the effect was bilateral. However, in one study the elderly activated right dorsal PFC when young subjects did not in a between-group comparison (Anderson et al., 2000) and in another study the elderly activated the left dorsal PFC when young subjects did not in within-group comparisons (Madden et al., 1999).
EM retrieval studies
Young subjects exhibited greater dorsal PFC activity compared to older subjects (4/9 studies; see Table 3), particularly in the right dorsal PFC. However, in three studies the elderly activated right dorsal PFC when young subjects did not (at the thresholds specified; Madden et al., 1999; Grady et al., 2002; Daselaar et al., 2003).
Summary
Across task domains both age groups activate the dorsal PFC. During WM tasks, older subjects exhibit greater left dorsal PFC activity compared to young. During EM encoding and retrieval young subjects recruited the bilateral dorsal PFC to a greater degree than older subjects; though this effect was stronger for EM encoding. The tabulated weighted sums of dorsal PFC activity across task domains demonstrates more reports of greater left dorsal PFC activity in older subjects compared to young (9 for young and 15 for older) and more reports of greater right dorsal PFC activity in the young compared to the older subjects (19 for young and 11 for the older). These results suggest that older subjects may under-recruit the right dorsal PFC and over-recruit the left dorsal PFC across tasks.
Anterior PFC
WM studies
Anterior PFC activity was reported during WM tasks in only four studies. Grady et al. (1998) found greater left anterior PFC activity in young subjects, but greater right anterior PFC activity in older subjects. Grossman et al. (2002) found greater bilateral anterior PFC in older subjects compared to young subjects. Smith et al. (2001) reported greater left anterior PFC activity in older versus younger subjects in the contrast comparing WM to control task activation. However, Smith et al. (2001) and Mitchell et al. (2000) reported greater right anterior PFC activity in young subjects. Thus, across experiments there was no consistent pattern in anterior PFC activity by age group.
EM encoding studies
In six studies reporting anterior PFC activity, three found greater left anterior PFC activity (Daselaar et al., 2003), and one found greater right anterior PFC activity (Grady et al., 1999), in the young versus the older subjects. However, Grady et al. (1999) reported greater left anterior PFC activity in older versus younger subjects for the level of processing contrast. In addition, another study (Morcom et al., 2002) reported greater bilateral anterior PFC activity in older versus young subjects during encoding.
EM retrieval studies
Overall, studies reported greater right versus left anterior PFC activity in young subjects during EM retrieval. In four of these studies older subjects under-recruited right anterior PFC compared to young subjects, for some of the task contrasts specified (Cabeza et al., 1997, 2000; Madden et al., 1999; Anderson et al., 2000).
However, Cabeza et al. (2002) reported greater bilateral anterior PFC activity in the elderly versus younger subjects during source memory retrieval versus cued recall, based on qualitative comparisons of within group analysis results. Two additional studies reported greater left anterior PFC activity in older subjects during EM retrieval (Madden et al., 1999; Cabeza et al., 2000).
Summary
Across task domains both age groups activate anterior PFC. The weighted total sums of reported age-related increases in left and right anterior activity indicate that there were greater reports of increased left anterior PFC activity, across task domains, in the older versus young subjects (8 for young and 12 for older). In contrast, there was greater right anterior PFC activity in the young versus the older subjects, across domains (10 for young and 7 for older).
Hemispheric differences in age-related deficits in PFC function
The weighted sum of the reported age-related increases tabulated for the bilateral ventral, dorsal and anterior PFC regions indicates that for all three regions there are fewer reports of right-lateralized activity for the elderly subjects compared to young subjects in the studies reviewed when one collapses across task domain (see bottom row of Tables 2–4). However, this effect is most evident in reports of dorsal PFC activations across task domains. In addition, the weighted sums of the reported age-related increases (tabulated for each PFC region) indicate that for all three subregions there are more reports of left-lateralized activity for the older compared to young subjects in the studies reviewed (see bottom row of Tables 2–4).
Summary of anterior PFC activations reported across studies
| Study . | Analytical methods . | Contrast . | Hemisphere . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Left . | . | Right . | . | ||||||
. | . | . | Young . | Old . | Young . | Old . | ||||||
| Working memory studies | ||||||||||||
| Grady et al. (1998) | Within-group univariate | WM > control | YY | OO | ||||||||
| Between-group multivariate | Long > short delay | Y | O | Y | O | |||||||
| Grossman et al. (2002) | Between-group univariate | Long > short linkage | OO | OO | ||||||||
| Mitchell et al. (2000) | Between-group ANOVA | Age X task interaction | YY | O | ||||||||
| Smith et al. (2001) | Within-group ROI analysis | Verbal WM > control | OO | |||||||||
| Within-group ROI analysis | Dual-task > single | YY | ||||||||||
| Episodic memory encoding studies | ||||||||||||
| Daselaar et al. (2003) | Within-group univariate SPM | Encoding > baseline | YY | |||||||||
| Grady et al. (1999) | Between-group multivariate PLS | Verbal > object encoding | YY | O | ||||||||
| Between-group multivariate PLS | Semantic > intentional encoding | YY | O | |||||||||
| Between-group multivariate PLS | Levels of processing effect | Y | OO | |||||||||
| Grady et al. (2002) | Between-group multivariate PLS | Encoding > baseline | YY | O | ||||||||
| Morcom et al. (2003) | Between-group univariate | Encoding > baseline related to subsequent memory | Y | OO | OO | |||||||
| Rosen et al. (2002) | Within-group univariate | Deep > shallow encoding | Y | O | ||||||||
| Schiavetto et al. (2002) | Between-group ANOVA | Encoding > Retrieval main effect and interaction | Y | O | ||||||||
| Episodic memory retrieval studies | ||||||||||||
| Anderson et al. (2000) | Between-group multivariate PLS | Retrieval > encoding | YY | YY | O | |||||||
| Cabeza et al. (2004) | Conjunction analysis | Retrieval > baseline | Y | O | ||||||||
| Cabeza et al. (2002) | Within-group univariate | Source memory > cued recall | OO | Y | OO | |||||||
| Cabeza et al. (2000) | Between-group ANOVA | Temporal order > item retrieval | YY | |||||||||
| Between-group ANOVA | Item > temporal order retrieval | OO | ||||||||||
| Cabeza et al. (1997) | Between-group multivariate PLS | Retrieval > encoding | YY | O | ||||||||
| Madden et al. (1999) | Within-group univariate | Retrieval > baseline | YY | |||||||||
| Between-group univariate | Retrieval > baseline | OO | ||||||||||
| Total sum of reported age-related increases | 8 | 12 | 10 | 7 | ||||||||
| Study . | Analytical methods . | Contrast . | Hemisphere . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Left . | . | Right . | . | ||||||
. | . | . | Young . | Old . | Young . | Old . | ||||||
| Working memory studies | ||||||||||||
| Grady et al. (1998) | Within-group univariate | WM > control | YY | OO | ||||||||
| Between-group multivariate | Long > short delay | Y | O | Y | O | |||||||
| Grossman et al. (2002) | Between-group univariate | Long > short linkage | OO | OO | ||||||||
| Mitchell et al. (2000) | Between-group ANOVA | Age X task interaction | YY | O | ||||||||
| Smith et al. (2001) | Within-group ROI analysis | Verbal WM > control | OO | |||||||||
| Within-group ROI analysis | Dual-task > single | YY | ||||||||||
| Episodic memory encoding studies | ||||||||||||
| Daselaar et al. (2003) | Within-group univariate SPM | Encoding > baseline | YY | |||||||||
| Grady et al. (1999) | Between-group multivariate PLS | Verbal > object encoding | YY | O | ||||||||
| Between-group multivariate PLS | Semantic > intentional encoding | YY | O | |||||||||
| Between-group multivariate PLS | Levels of processing effect | Y | OO | |||||||||
| Grady et al. (2002) | Between-group multivariate PLS | Encoding > baseline | YY | O | ||||||||
| Morcom et al. (2003) | Between-group univariate | Encoding > baseline related to subsequent memory | Y | OO | OO | |||||||
| Rosen et al. (2002) | Within-group univariate | Deep > shallow encoding | Y | O | ||||||||
| Schiavetto et al. (2002) | Between-group ANOVA | Encoding > Retrieval main effect and interaction | Y | O | ||||||||
| Episodic memory retrieval studies | ||||||||||||
| Anderson et al. (2000) | Between-group multivariate PLS | Retrieval > encoding | YY | YY | O | |||||||
| Cabeza et al. (2004) | Conjunction analysis | Retrieval > baseline | Y | O | ||||||||
| Cabeza et al. (2002) | Within-group univariate | Source memory > cued recall | OO | Y | OO | |||||||
| Cabeza et al. (2000) | Between-group ANOVA | Temporal order > item retrieval | YY | |||||||||
| Between-group ANOVA | Item > temporal order retrieval | OO | ||||||||||
| Cabeza et al. (1997) | Between-group multivariate PLS | Retrieval > encoding | YY | O | ||||||||
| Madden et al. (1999) | Within-group univariate | Retrieval > baseline | YY | |||||||||
| Between-group univariate | Retrieval > baseline | OO | ||||||||||
| Total sum of reported age-related increases | 8 | 12 | 10 | 7 | ||||||||
This table presents reviewed studies that reported anterior PFC activity. Reports of anterior PFC activity are listed first by study type (first column; WM, for working memory anterior PFC activations are presented in columns two and three, respectively. The activations are organized by cerebral hemisphere (Left versus Right) and by age group (Young versus Old). Y's and O's are used to notate the activations reported in Young and Old subject groups across studies, respectively. Y, anterior PFC activity in young subjects; O, anterior PFC activity in old subjects; YY, the authors reported young only or young greater than old activity (Y > O) in this region for a specific task effect. OO, the authors reported old only or old greater than young (O > Y) activity in this region for a specific task effect. The bottom row of the table represents the total weighted sum of reported left/right anterior PFC activation in young or old subjects across the studies presented (refer to the Methods section to see how the sum was calculated).
Summary of anterior PFC activations reported across studies
| Study . | Analytical methods . | Contrast . | Hemisphere . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Left . | . | Right . | . | ||||||
. | . | . | Young . | Old . | Young . | Old . | ||||||
| Working memory studies | ||||||||||||
| Grady et al. (1998) | Within-group univariate | WM > control | YY | OO | ||||||||
| Between-group multivariate | Long > short delay | Y | O | Y | O | |||||||
| Grossman et al. (2002) | Between-group univariate | Long > short linkage | OO | OO | ||||||||
| Mitchell et al. (2000) | Between-group ANOVA | Age X task interaction | YY | O | ||||||||
| Smith et al. (2001) | Within-group ROI analysis | Verbal WM > control | OO | |||||||||
| Within-group ROI analysis | Dual-task > single | YY | ||||||||||
| Episodic memory encoding studies | ||||||||||||
| Daselaar et al. (2003) | Within-group univariate SPM | Encoding > baseline | YY | |||||||||
| Grady et al. (1999) | Between-group multivariate PLS | Verbal > object encoding | YY | O | ||||||||
| Between-group multivariate PLS | Semantic > intentional encoding | YY | O | |||||||||
| Between-group multivariate PLS | Levels of processing effect | Y | OO | |||||||||
| Grady et al. (2002) | Between-group multivariate PLS | Encoding > baseline | YY | O | ||||||||
| Morcom et al. (2003) | Between-group univariate | Encoding > baseline related to subsequent memory | Y | OO | OO | |||||||
| Rosen et al. (2002) | Within-group univariate | Deep > shallow encoding | Y | O | ||||||||
| Schiavetto et al. (2002) | Between-group ANOVA | Encoding > Retrieval main effect and interaction | Y | O | ||||||||
| Episodic memory retrieval studies | ||||||||||||
| Anderson et al. (2000) | Between-group multivariate PLS | Retrieval > encoding | YY | YY | O | |||||||
| Cabeza et al. (2004) | Conjunction analysis | Retrieval > baseline | Y | O | ||||||||
| Cabeza et al. (2002) | Within-group univariate | Source memory > cued recall | OO | Y | OO | |||||||
| Cabeza et al. (2000) | Between-group ANOVA | Temporal order > item retrieval | YY | |||||||||
| Between-group ANOVA | Item > temporal order retrieval | OO | ||||||||||
| Cabeza et al. (1997) | Between-group multivariate PLS | Retrieval > encoding | YY | O | ||||||||
| Madden et al. (1999) | Within-group univariate | Retrieval > baseline | YY | |||||||||
| Between-group univariate | Retrieval > baseline | OO | ||||||||||
| Total sum of reported age-related increases | 8 | 12 | 10 | 7 | ||||||||
| Study . | Analytical methods . | Contrast . | Hemisphere . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | Left . | . | Right . | . | ||||||
. | . | . | Young . | Old . | Young . | Old . | ||||||
| Working memory studies | ||||||||||||
| Grady et al. (1998) | Within-group univariate | WM > control | YY | OO | ||||||||
| Between-group multivariate | Long > short delay | Y | O | Y | O | |||||||
| Grossman et al. (2002) | Between-group univariate | Long > short linkage | OO | OO | ||||||||
| Mitchell et al. (2000) | Between-group ANOVA | Age X task interaction | YY | O | ||||||||
| Smith et al. (2001) | Within-group ROI analysis | Verbal WM > control | OO | |||||||||
| Within-group ROI analysis | Dual-task > single | YY | ||||||||||
| Episodic memory encoding studies | ||||||||||||
| Daselaar et al. (2003) | Within-group univariate SPM | Encoding > baseline | YY | |||||||||
| Grady et al. (1999) | Between-group multivariate PLS | Verbal > object encoding | YY | O | ||||||||
| Between-group multivariate PLS | Semantic > intentional encoding | YY | O | |||||||||
| Between-group multivariate PLS | Levels of processing effect | Y | OO | |||||||||
| Grady et al. (2002) | Between-group multivariate PLS | Encoding > baseline | YY | O | ||||||||
| Morcom et al. (2003) | Between-group univariate | Encoding > baseline related to subsequent memory | Y | OO | OO | |||||||
| Rosen et al. (2002) | Within-group univariate | Deep > shallow encoding | Y | O | ||||||||
| Schiavetto et al. (2002) | Between-group ANOVA | Encoding > Retrieval main effect and interaction | Y | O | ||||||||
| Episodic memory retrieval studies | ||||||||||||
| Anderson et al. (2000) | Between-group multivariate PLS | Retrieval > encoding | YY | YY | O | |||||||
| Cabeza et al. (2004) | Conjunction analysis | Retrieval > baseline | Y | O | ||||||||
| Cabeza et al. (2002) | Within-group univariate | Source memory > cued recall | OO | Y | OO | |||||||
| Cabeza et al. (2000) | Between-group ANOVA | Temporal order > item retrieval | YY | |||||||||
| Between-group ANOVA | Item > temporal order retrieval | OO | ||||||||||
| Cabeza et al. (1997) | Between-group multivariate PLS | Retrieval > encoding | YY | O | ||||||||
| Madden et al. (1999) | Within-group univariate | Retrieval > baseline | YY | |||||||||
| Between-group univariate | Retrieval > baseline | OO | ||||||||||
| Total sum of reported age-related increases | 8 | 12 | 10 | 7 | ||||||||
This table presents reviewed studies that reported anterior PFC activity. Reports of anterior PFC activity are listed first by study type (first column; WM, for working memory anterior PFC activations are presented in columns two and three, respectively. The activations are organized by cerebral hemisphere (Left versus Right) and by age group (Young versus Old). Y's and O's are used to notate the activations reported in Young and Old subject groups across studies, respectively. Y, anterior PFC activity in young subjects; O, anterior PFC activity in old subjects; YY, the authors reported young only or young greater than old activity (Y > O) in this region for a specific task effect. OO, the authors reported old only or old greater than young (O > Y) activity in this region for a specific task effect. The bottom row of the table represents the total weighted sum of reported left/right anterior PFC activation in young or old subjects across the studies presented (refer to the Methods section to see how the sum was calculated).
Discussion
The goal of this review was to determine whether there are region-specific changes in PFC function with age. A comprehensive review of published human functional neuroimaging studies revealed activation foci in the bilateral ventral, dorsal and anterior PFC regions across WM, EM encoding and EM retrieval tasks, in both age groups. By examining age-related differences within each task domain, we found that overall both age groups engage the similar brain regions while performing WM and EM tasks. This suggests that in general PFC function remains intact with age. However, by tabulating age-related differences in PFC activity across task domains we found that there were region-specific changes in PFC function with age. Thus, we argue that strict adherence to either the traditional functional compensation or dedifferentiation perspectives cannot adequately account for the complex patterns of age-related changes observed, since neither of these perspectives takes into account the differential patterns of region-specific, age-related change in prefrontal function (Nielsen-Bohlman and Knight, 1995; Li et al., 2001; Braver and Barch, 2002; Cabeza, 2002; Grady, 2002; Reuter-Lorenz, 2002).
In the following sections, we discuss how the age-related changes in functional activation in the ventral, dorsal and anterior PFC support the hypothesis that there are region-specific changes in PFC function with age. In addition, we present hypotheses of what these distinct region-specific changes in function may reflect on a neural level: dedifferentiation of cortical function, deficits in cortical function or functional compensation, respectively. However, to dissociate these underlying neural changes we must first present criteria for distinguishing between them.
Primary deficits in function refers to age-related neural dysfunction and would result in region-specific decreases in activity across all tasks with concomitant decreases in behavioural performance and brain-behaviour relationships in only older subjects. For example, Cabeza et al. (2000) observed right anterior PFC activity in young subjects, but not older subjects, during a PET study of temporal order memory. Older subjects also exhibited performance deficits during temporal order memory tasks. These results suggest that there is a primary deficit in right anterior PFC function with age.
Age-related functional compensation refers to increases in non-task-related brain regions, which results in better behavioural performance in older subjects. Task-related brain regions are those that are engaged during task performance in young subjects and non-task-related brain regions are those areas that are uniquely engaged in older subjects. Functional compensation would result in activity in both task-related and non-task-related brain regions in older subjects; however, activity in task-related brain regions may be reduced in older subjects compared to young subjects. In addition, activity in both task-related and non-task-related brain regions should produce concomitant increases in behavioural performance and brain-behaviour relationships in older subjects, though these effects may be lower in task-related brain regions.
It should, however, be noted that age-related increases in PFC activity that reflect functional compensation may not always produce performance benefits and could be interpreted as reflecting attempted functional compensation. In fact, one could argue that all increases in brain activity with age reflect the ageing brain's attempts to functionally compensate for primary deficits in function in the other PFC and posterior cortical regions. However, such an extreme argument ignores the equally plausible idea that the ageing brain is a functionally noisier system (Huettel et al., 2001; Li et al., 2001; D'Esposito et al., 2003), and hence increases in the PFC may reflect spurious non-task related activity, perhaps due to dedifferentiation in function (see below). These non-task related increases in brain activity with age would not reflect attempted/successful compensation and thus would not be related to task performance. Due to this latter alternate explanation for age-related increases in activity that are not related to task performance, in the current review we only classify age-related activity increases in non-task-related PFC regions as reflecting functional compensation if there was a concomitant performance benefit in the elderly.
Age-related dedifferentiation of function refers to decreases in signal-to-noise ratio in cortical processing resulting in reduced regional process-specificity and increases in non-specific cortical activation across tasks, with age (Li et al., 2001). This would result in: (i) reduced activity in task-related brain regions with possible reductions in brain-behaviour correlations in older subjects, compared to young subjects and (ii) generalized activity of cortical regions in a non-selective manner, across tasks, which does not correlate well with behaviour, in older subjects alone (Logan et al., 2002). Thus, in this review of the published literature, by examining regional activations across task domains and their correlations with the respective behavioural output, we present hypotheses as to whether age-related changes in cortical function reflect dysfunction, functional compensation, or dedifferentiation. We hope that investigators attempt to test these hypotheses directly in the future.
Region-specific changes in PFC function with age
Ventral PFC activity: dedifferentiation or failed attempt at compensation?
We found that there were differences in ventral PFC activity with age. Similar to young subjects, older subjects activate the ventral PFC, particularly in the left hemisphere, during WM and EM encoding tasks. However, the elderly activate this region to a lesser degree than young subjects during these tasks. In contrast, the elderly over-recruit this region during EM retrieval compared to young subjects. Therefore, elderly subjects tend to activate the ventral PFC across task domains, in a less task-specific manner, compared to young subjects.
In studies that performed brain-behaviour analyses during WM and EM encoding tasks, different relationships for young versus older subjects were found. For example, in a WM task, Grady et al. (1998) found that better WM performance was related to increased right ventral PFC activity in young subjects, but not older subjects. During EM tasks, both Grady et al. (2002) and Morcom et al. (2003) found that left ventral PFC activity during encoding was related to higher subsequent memory effects in both age groups. These results together, particularly the EM encoding brain-behaviour results, show that ventral PFC activity is positively related to task performance in both age groups indicating that the reduced left ventral PFC activity in the elderly is not reflective of a primary deficit in function.
Logan et al. (2002) have shown that the age-related under-recruitment of left ventral PFC during EM encoding is related to failure of the elderly to spontaneously engage effective encoding strategies; however, when effective strategies are given to them, the elderly do not under-recruit this region. This finding also supports the view that reduced left ventral PFC activity in the elderly is not indicative of a primary deficit, but reflects inadequate automatic use of encoding strategies under normal conditions possibly due to reduced availability of processing resources (Craik, 1982; Craik and Byrd, 1982; Li et al., 2001; Lindenberger et al., 2001). We hypothesize that on a neural level this may be due to increased noise in the system (dedifferentiation) with age.
In some conditions, such as EM retrieval, older subjects activate the ventral PFC, particularly the left ventral PFC, to a greater degree than younger subjects (see Table 2) and this over-recruitment has been interpreted as reflecting functional compensation at a neural level (Cabeza, 2002; Park et al., 2003). However, this over-recruitment does not correlate well with retrieval behaviour (Cabeza et al., 1997, 2002). Also, in the three EM retrieval studies that reported greater left ventral PFC activity in the older versus younger subjects (Madden et al., 1999; Anderson et al., 2000; Cabeza, 2002), the elderly still performed worse at retrieval compared to young. Together these results suggest that over-recruitment of the left ventral PFC at retrieval may not be compensatory in nature but may reflect non-selective activity.
Alternatively, the increased EM retrieval-related activity in the ventral PFC with age may reflect a failed attempt of the ageing brain to compensate for deficits in other PFC regions during retrieval. In the study by Logan et al. (2002) when encoding strategies were given to the elderly, they activate the left ventral PFC during encoding in a similar way to young subjects. Perhaps under intentional encoding and subsequent retrieval conditions, the elderly fail to use appropriate strategies at encoding and try to compensate by using these strategies at retrieval and thus activate the left ventral PFC at retrieval. However, given that current evidence does not suggest that neither the EM encoding related under-recruitment nor the EM retrieval related over-recruit of the left ventral PFC were directly detrimental or beneficial to behaviour, respectively, it is unclear how the neural mechanisms of primary deficit and/or functional compensation could underlie this pattern of ventral PFC activity in the elderly.
Instead, this pattern could be reflective of dedifferentiation of function with age, which thus results in non-selective activation of the left ventral PFC across task domains due to reduced representational specialization on a cortical level (Li et al., 2001; Lindenberger et al., 2001; Logan et al., 2002; Park et al., 2004). In a recent fMRI study by Park et al. (2004) an age-related reduction in neural specialization in ventral visual regions dedicated to processing faces, places and words, was observed, and taken to support the dedifferentiation hypothesis for ventral visual cortical function with age. Considering that information from the ventral visual cortex projects to the ventral PFC provides additional support for the hypothesis that age-related changes in ventral PFC function may be reflective of neural dedifferentiation (Ungerleider and Mishkin, 1982; Haxby et al., 1988, 1993; Ungerleider et al., 1989).
Support for the dedifferentiation hypothesis of ventral PFC function also comes from recent in vivo volumetric studies by Tisserand et al. (2002, 2004), which report that the strongest age-related grey matter volume reduction in the PFC was in the bilateral ventral PFC (i.e. inferior frontal gyrus); moreover, volume loss in this region was greater in older subjects who exhibited poorer performance during neuropsychological assessments (Tisserand et al., 2004). Greater age-related atrophy in inferior frontal gyrus was also observed by Good et al. (2001) using voxel-based morphometry. Therefore, this age-related decrease in bilateral ventral PFC grey matter may result in, or, be the result of, neural dedifferentiation of function within this region with age.
Together, these observations present a strong argument for dedifferentiation of the ventral PFC, particularly in the left hemisphere. However, it is still possible that this pattern of activity is reflective of compensation, albeit failed compensation. One of the future challenges for researchers is to test these competing hypotheses.
Left dorsal and anterior PFC: functional compensation?
In this review we found greater age-related increases in left versus right activation in the PFC, across task domains (Cabeza et al., 1997, 2004; Grady et al., 1998, 2002; Madden et al., 1999; Anderson et al., 2000; Smith et al., 2001; Grossman et al., 2002; Schiavetto et al., 2002; Daselaar et al., 2003). Most of these increases were observed in left dorsal and anterior PFC.
The left dorsal PFC was recruited by older subjects to a greater degree across task domains compared to young subjects, suggesting that left dorsal activity in older subjects may reflect functional compensation. However, the brain-behaviour analyses conducted to date, do not present strong evidence for this interpretation. For example, Daselaar et al. (2003) report similar left dorsal PFC activity in young, high and low-performing older subjects during EM retrieval whereas Cabeza et al. (2002) report greater left dorsal PFC activity in young versus high and low-performing older subjects. Also, Grady et al. (1998) reported that increased left dorsal PFC activity in older subjects was correlated with longer reaction times during a WM task. Thus, it is unclear whether age-related increases in the left dorsal PFC reflect functional compensation. Perhaps, these age-related increases reflect the attempt of ageing brains to compensate for functional deficits in the right PFC, which at times is effective.
There is stronger support that the age-related increase in the left anterior PFC is reflective of functional compensation. First, studies have shown increased left anterior PFC in older subjects compared to young subjects, across task domains (Madden et al., 1999; Cabeza et al., 2000, 2002; Smith et al., 2001; Grossman et al., 2002; Morcom et al., 2003). Second, studies have also found that this increase is related to better task performance in the older subjects. For example, Morcom et al. (2003) and Cabeza et al. (2002) reported increased left anterior PFC activity only in older subjects and this activity was related to better task performance at EM retrieval. Also, Cabeza et al. (2002) found that during EM retrieval, young, high and low-performing older subjects activate the right anterior PFC, but only high-performing older subjects activate the left anterior PFC.
A recent neuroimaging study in young subjects (Koechlin, 2003) and connectionist models (O'Reilly et al., 2002) have posited a model of hierarchical PFC organization. In the fMRI study (Koechlin, 2003), manipulation of the number of responses in the task affected premotor cortex activity, manipulation of the number of relevant stimulus dimensions affected dorsal PFC activity and manipulation of the across-block frequency of cue-to-response or cue-to-dimension mappings affected anterior PFC activity. Interestingly, structural equation modelling of the fMRI data revealed path coefficients from anterior PFC to dorsal PFC to premotor cortex, which is broadly consistent with a hierarchic organization. Though this model does not directly test, nor support, a ventral-to-dorsal-to-anterior PFC hierarchy, the results still suggest that there is a hierarchical pattern of information flow from posterior to anterior regions within the PFC, which in turn places anterior PFC at the top of the hierarchy, and in a position to be recruited when ‘lower level’ systems fail. Therefore, one possible interpretation of the current review results is that with age there may be dedifferentiation of function in the left ventral PFC resulting in compensatory recruitment of the left dorsal and anterior PFC. Such a hypothesis of left PFC changes with age is consistent with the hierarchical model proposed by Koechlin et al. (2003) and can be easily tested in future studies.
Changes in right dorsal and anterior PFC: deficits in function?
Previous neuroimaging studies of cognitive ageing have consistently reported age-related decreases in right dorsal and anterior PFC activity (Cabeza, 1997, 2002; Grady et al., 1999, 2002; Anderson et al., 2000; Rypma and D'Esposito, 2000; Smith et al., 2001). Across WM and EM studies, older subjects either fail to engage these regions at the threshold specified or do not engage these areas to the same degree as young subjects. Additionally, the weighted total sums of activations across task domains indicate that studies have reported less right dorsal and anterior PFC activity in older versus young subjects. Brain-behaviour analyses indicate that when the elderly do activate the right dorsal PFC it does not aid task-performance. For example, studies have shown that increased right dorsal PFC activity is directly related to poorer behavioural performance in older subjects but not younger subjects (Madden et al., 1999; Rypma and D'Esposito, 2000). Moreover, right dorsal PFC activity was observed in both young and low performing-older subjects, but not high-performing older subjects (Cabeza, 2002). This suggests that ageing disrupts the normal functioning of these brain regions.
Together these results support the right hemi-ageing hypothesis which proposes that there is greater right- versus left- lateralized functional decline in the cerebral hemispheres with age (Brown and Jaffe, 1975; Lapidot, 1987; Albert and Moss, 1988; Cermak et al., 1989; Ellis and Oscar-Berman, 1989; McDowell et al., 1994; Gerhardstein et al., 1998; Dolcos et al., 2002). This hypothesis is supported by a variety of behavioural studies of verbal versus spatial processing, dichotic listening, object processing of tachistocopically presented stimuli, sensorimotor processing and emotional processing (see Dolcos et al., 2002 for a review). For example, Gerhardstein et al. (1998) examined age differences in judgements of similarity between two objects presented sequentially to either the left visual field (right hemisphere) or right visual field (left hemisphere) and found that older subjects exhibited greater deficits in similarity judgements for stimuli presented in the left visual field. Moreover, neuropsychological studies have shown that older subjects perform similarly to patients with right, but not left, hemispheric damage during prosodic perception (Orbelo et al., 2003), strategy application (Levine et al., 1998) and word-list learning (Stuss et al., 1996; Mangels, 1997).
Importantly, the right hemi-ageing hypothesis does not explicitly propose a region-specific right hemisphere decline in the PFC. However, our review suggests that there is a region-specific change within the right dorsal and anterior PFC during WM and EM tasks. Thus, the results of this review and that of previous studies supporting the right hemi-ageing hypothesis raise the following question: why would the right cerebral hemisphere, and the right dorsal and anterior PFC particularly, be more vulnerable to age-related decline? To date, there has not been a single study, to our knowledge, that has directly examined possible underlying causes for the lateralised age-related decline of right versus left frontal cortex. However, volumetric and psychopharmacological studies have indirectly found that right frontal hemi-ageing may result from hemispheric differences in the maturational trajectories of the cerebral hemispheres, grey/white matter ratios, and the lateralization of various neurotransmitter systems, especially of dopamine (Oke et al., 1978; Slopsema et al., 1982; Mann, 1983; Arnsten and Goldman-Rakic, 1987; Morgan, 1987; van Dyck et al., 1995, 2002; Riekkinen et al., 1996; Barili et al., 1998; Larisch et al., 1998; Baltes et al., 1999; Miguez et al., 1999; Mohr et al., 2003; Raz et al., 2004; Sowell et al., 2004). For example, volumetric studies have shown that the right hemisphere exhibits a steeper maturational slope and by adulthood the right hemisphere has greater volume than the left hemisphere, particularly in the frontal lobes (Watkins et al., 2001; Lee et al., 2004; Raz et al., 2004; Sowell et al., 2004). In contrast, there is an age-related increase in grey matter density within the left hemisphere between adolescence and adulthood compared to the right hemisphere and studies have reported a larger grey-to-white matter ratio in specific left hemispheric regions compared to the right (Watkins et al., 2001; Sowell et al., 2004). Therefore, these volumetric studies indicate that there is basic structural difference between hemispheres, which may underlie the right hemispheric deficits observed in the dorsal and anterior PFC with age; however, more research is required to clarify this relationship.
Age-related changes in the neurotransmitter systems may also underlie the lateralized decline of the right versus left PFC that has been observed across neuroimaging, neuropsychological and behavioural studies. Pharmacological studies have found hemispheric lateralization of dopamine, serotonin, and norepinephrine neurotransmitter systems in distinct brain regions (Oke et al., 1978; Nowak, 1989; Rodriguez et al., 1994; Larisch et al., 1998; Andersen and Teicher, 1999; de la Fuente-Fernandez et al., 2000; Mohr et al., 2003). Lateralisation effects within the dopamine system are of particular interest when investigating potential causes for right hemi-ageing of the PFC since there is age-related decline in pre-synaptic markers and post-synaptic D1 and D2 receptor subtypes within the striatum and frontal cortex; moreover, age-related decline in D2 receptor binding has been related to associated deficits in cognitive and motor task performance (McGeer, 1981; Morgan, 1987; Volkow et al., 1998a, 1998b, 2000; Miguez et al., 1999; de la Fuente-Fernandez et al., 2000; Braver and Barch, 2002; van Dyck et al., 2002). Larisch et al. (1998) have reported greater right versus left D2 receptor binding within the striatum of rats. Clearly further investigation is required to confirm the possibility that there is a lateralization of the dopamine system within the frontal cortex, however the current data suggest that right hemi-ageing of the dorsal and anterior PFC may be due to hemispheric asymmetries in dopamine receptor subtypes and concomitant age-related declines in dopamine function.
Conclusions
By reviewing age-related differences in PFC activity across WM and EM studies, and collapsing across tasks, we found that there were region-specific changes in PFC function with age. There were both age-related decreases and increases in specific PFC regions. We hypothesize from the activation patterns of these distinct PFC regions in young versus older subjects and the brain-behaviour relationships reported across studies, that with age there are deficits in function, dedifferentiation of function, and functional compensation co-occurring in distinct PFC regions. It is possible that dedifferentiation leads to primary deficits in function and that these two functional changes may reflect different points on a continuum of age-related neural degeneration due to grey and white matter loss, dendritic regression and neurotransmitter deficits (Raz, 2000; Cabeza et al., 2005). Alternatively, dedifferentiation and deficits in function may be symptomatic of different types of underlying structural and/or neurotransmitter changes with age. Within this framework, region-specific functional decline, due to either dedifferentiation or deficits in function, in turn causes the ageing brain to attempt to compensate in a region-specific manner (Buckner, 2004; Cabeza et al., 2005).
Building upon this framework, we propose the following region-specific model of ageing that we hope will stimulate future research: due to neural degeneration and changes in neurotransmitter systems, with normal ageing, there results dedifferentiation of cortical function in the bilateral ventral PFC and deficits in function in the right dorsal and anterior PFC. These changes in turn result in functional compensation within the left dorsal and anterior PFC. We hope that future studies in the cognitive neuroscience of ageing examine regional changes in PFC sub-regions across task domains, and correlate these regional changes with behavioural performance. By doing so, these studies will be able to corroborate, modify or refute the hypotheses drawn from the current review.
Thank you to C. Lehrer and L. Mason for help in preparation of this manuscript and to A. Gazzaley and J. Rissman for their feedback. This work was supported by the National Institutes of Health and the American Federation for Aging Research.
References
Andersen SL, Teicher MH. Serotonin laterality in amygdala predicts performance in the elevated plus maze in rats.
Anderson ND, Iidaka T, Cabeza R, Kapur S., McIntosh AR, Craik FI. The effects of divided attention on encoding- and retrieval-related brain activity: a PET study of younger and older adults.
Andres P. Frontal cortex as the central executive of working memory: time to revise our view.
Arnsten AF, Goldman-Rakic PS. Noradrenergic mechanisms in age-related cognitive decline.
Baltes PB, Staudinger UM, Lindenberger U. Lifespan psychology: theory and application to intellectual functioning.
Barili P, De Carolis G, Zaccheo D, Amenta F. Sensitivity to ageing of the limbic dopaminergic system: a review.
Bechara A. The role of emotion in decision-making: evidence from neurological patients with orbitofrontal damage.
Bonin GV, Bailey P. The neocortex of the Macaca mulatta. Urbana, IL: University of Illinois Press;
Braver TS, Barch DM. A theory of cognitive control, aging cognition, and neuromodulation.
Brodmann K. Vergleichende Lokalisationslehre der Großhirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig: Barth JA;
Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate.
Buckner RL, Snyder AZ, Sanders AL, Raichle ME, Morris JC. Functional brain imaging of young, nondemented, and demented older adults.
Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model.
Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies.
Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, et al. Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study.
Cabeza R, Anderson ND, Houle S, Mangels JA, Nyberg L. Age-related differences in neural activity during item and temporal-order memory retrieval: a positron emission tomography study.
Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults.
Cabeza R, Locantore JK, Anderson ND. Lateralization of prefrontal activity during episodic memory retrieval: evidence for the production-monitoring hypothesis.
Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval.
Cabeza R, Nyberg L, Park DC, editors. Cognitive neuroscience of aging. New York: Oxford University Press;
Campisi J. Cellular senescence and apoptosis: how cellular responses might influence aging phenotypes.
Carmichel ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys.
Castner SA, Goldman-Rakic PS, Williams GV. Animal models of working memory: insights for targeting cognitive dysfunction in schizophrenia.
Cermak LS, Verfaellie M, Letourneau L, Blackford S, Weiss S, Numan B. Verbal and nonverbal right hemisphere processing by chronic alcoholics.
Chao LL, Knight RT. Prefrontal deficits in attention and inhibitory control with aging.
Collins P, Roberts AC, Dias R, Everitt BJ, Robbins TW. Perseveration and strategy in a novel spatial self-ordered sequencing task for nonhuman primates: effects of excitotoxic lesions and dopamine depletions of the prefrontal cortex.
Craik FIM. Selective changes in encoding as a function of reduced processing capacity. In: Klix F, Hoffmann J, van der Meer E, editors. Cognitive research in psychology. Berlin: Deutschen Verlag der Wissenschaften;
Craik FIM, Byrd M. Aging and cognitive deficits: The role of attentional resources. In: Craik FIM, Trehub S, editors. Aging and cognitive processes. New York: Plenum Press;
Craik FIM, Grady CL. Aging, memory and frontal lobe functioning. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York City, NY: Oxford University Press;
Craik FIM, Salthouse TA, editors. The handbook of aging and cognition, 2nd ed. Mahwah, NJ: Lawrence Erlbaum Associates;
Curtis C, D'Esposito M. Success and failure suppressing reflexive behaviour.
Daselaar SM, Veltman DJ, Rombouts SA, Raaijmakers JG, Jonker C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects.
de Brabander JM, Kramers RJ, Uylings HB. Layer-specific dendritic regression of pyramidal cells with ageing in the human prefrontal cortex.
de la Fuente-Fernandez R, Kishore A, Calne DB, Ruth TJ, Stoessl AJ. Nigrostriatal dopamine system and motor lateralization.
Della-Maggiore V, Sekuler AB, Grady CL, Bennett PJ, Sekuler R, McIntosh AR. Corticolimbic interactions associated with performance on a short-term memory task are modified by age.
Della-Maggiore V, Grady CL, McIntosh AR. Dissecting the effect of aging on the neural substrates of memory: deterioration, preservation or functional reorganization?
D'Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory.
D'Esposito M, Zarahn E, Aguirre GK, Rypma B. The effect of normal aging on the coupling of neural activity to the bold hemodynamic response.
D'Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: an event-related fMRI study.
D'Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies.
D'Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging.
Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts.
Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: restriction to novel situations and independence from `on-line' processing.
Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: multiple prefrontal processes subserve source memory.
Dobbins IG, Rice HJ, Wagner AD, Schacter DL. Memory orientation and success: separable neurocognitive components underlying episodic recognition.
Dobbins IG, Simons JS, Schacter DL. fMRI evidence for separable and lateralized prefrontal memory monitoring processes.
Dolcos F, Rice HJ, Cabeza R. Hemispheric asymmetry and aging: right hemisphere decline or asymmetry reduction.
Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands.
Economo C, Koskinas GN. Die Cytoarchitectonik der Hirnrinde des Erwachsenen Menschen. Berlin, Germany: Verlag von Julius Springer;
Ellis RJ, Oscar-Berman M. Alcoholism, aging, and functional cerebral asymmetries.
Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm.
Fletcher PC, Frith CD, Rugg MD. The functional neuroanatomy of the episodic memory.
Gabrieli JD. Memory systems analyses of mnemonic disorders in aging and age-related diseases.
Gazzaley A, D'Esposito M. The contribution of functional brain imaging to our understanding of cognitive aging.
Gazzaniga MS. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition?
Gerhardstein P, Peterson MA, Rapcsak SZ. Age-related hemispheric asymmetry in object discrimination.
Gilboa A. Autobiographical and episodic memory—one and the same? Evidence from prefrontal activation in neuroimaging studies.
Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains.
Gould RL, Brown RG, Owen AM, Ffytche DH, Howard RJ. fMRI BOLD response to increasing task difficulty during successful paired associates learning.
Grachev ID, Apkarian AV. Aging alters regional multichemical profile of the human brain: an in vivo 1H-MRS study of young versus middle-aged subjects.
Grady CL. Age-related differences in face processing: a meta-analysis of three functional neuroimaging experiments.
Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, et al. Age-related changes in cortical blood flow activation during visual processing of faces and location.
Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, et al. Age-related reductions in human recognition memory due to impaired encoding.
Grady CL, McIntosh AR, Bookstein F, Horwitz B, Rapoport SI, Haxby JV. Age-related changes in regional cerebral blood flow during working memory for faces.
Grady CL, McIntosh AR, Rajah MN, Beig S, Craik FIM. The effects of age on the neural correlates of episodic encoding.
Grady CL, Bernstein LJ, Beig S, Siegenthaler AL. The effects of encoding task on age-related differences in the functional neuroanatomy of face memory.
Grossman M, Cooke A, DeVita C, Alsop D, Detre J, Chen W, et al. Age-related changes in working memory during sentence comprehension: an fMRI study.
Habib R, Nyberg L, Tulving E. Hemispheric asymmetries of memory: the HERA model revisited.
Haxby JV, Grady CL, Horwitz B, Schapiro MB, Carson RE, Ungerleider LG, et al. Mapping of two visual pathways in man with regional cerebral blood flow (rCBF) as measured by positron emission tomography (PET) and H215O.
Haxby JV, Grady CL, Horwitz B, Salerno JA, Ungerleider LG, Mishkin M, et al. Dissociation of object and spatial visual processing pathways in human extrastriate cortex. In: Gulyas B, Ottoson D, Roland PE, editors. Functional organization of human visual cortex. Oxford: Pergamon Press;
Hazlett EA, Buchsbaum MS, Mohs RC, Spiegel-Cohen J, Wei TC, Azueta R, et al. Age-related shift in brain region activity during successful memory performance.
Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study.
Hesselmann V, Zaro Weber O, Wedekind C, Krings T, Schulte O, Kugel H, et al. Age related signal decrease in functional magnetic resonance imaging during motor stimulation in humans.
Huettel SA, Singerman JD, McCarthy G. The effects of aging upon the hemodynamic response measured by functional MRI.
Iidaka T, Okada T, Murata T, Omori M, Kosaka H, Sadato N, et al. Age-related differences in the medial temporal lobe responses to emotional faces as revealed by fMRI.
Johnson MK, Mitchell KJ, Raye CL, Greene EJ. An age-related deficit in prefrontal cortical function associated with refreshing information.
Jonides J, Marshuetz C, Smith EE, Reuter-Lorenz PA, Koeppe RA, Hartley A. Age differences in behavior and PET activation reveal differences in interference resolution in verbal working memory.
Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex.
Labat-Robert J. Cell-matrix interactions, alteration with aging and age associated diseases. A review.
Ladislas R. Cellular and molecular mechanisms of aging and age related diseases.
Langley LK, Madden DJ. Functional neuroimaging of memory: implications for cognitive aging.
Lapidot MB. Does the brain age uniformly? Evidence from effects of smooth pursuit eye movements on verbal and visual tasks.
Larisch R, Meyer W, Klimke A, Kehren F, Vosberg H, Muller-Gartner HW. Left-right asymmetry of striatal dopamine D2 receptors.
Lee JM, Yoon U, Kim JJ, Kim IY, Lee DS, Kwon JS, et al. Analysis of the hemispheric asymmetry using fractal dimension of a skeletonized cerebral surface.
Levine B, Stuss DT, Milberg WP, Alexander MP, Schwartz M, Macdonald R. The effects of focal and diffuse brain damage on strategy application: evidence from focal lesions, traumatic brain injury and normal aging.
Li SC, Lindenberger U, Sikstrom S. Aging cognition: from neuromodulation to representation.
Lindenberger U, Scherer H, Baltes PB. The strong connection between sensory and cognitive performance in old age: not due to sensory acuity reductions operating during cognitive assessment.
Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging.
Lustig C, Buckner RL. Preserved neural correlates of priming in old age and dementia.
MacPherson SE, Phillips LH, Della Sala S. Age, executive function, and social decision making: a dorsolateral prefrontal theory of cognitive aging.
Madden DJ, Turkington TG, Provenzale JM, Denny LL, Hawk TC, Gottlob LR, et al. Adult age differences in the functional neuroanatomy of verbal recognition memory.
Madden DJ, Langley LK, Denny LL, Turkington TG, Provenzale JM, Hawk TC, et al. Adult age differences in visual word identification: functional neuroanatomy by positron emission tomography.
Mangels JA. Strategic processing and memory for temporal order in patients with frontal lobe lesions.
Mann DM. The locus coeruleus and its possible role in ageing and degenerative disease of the human central nervous system.
McDowell CL, Harrison DW, Demaree HA. Is right hemisphere decline in the perception of emotion a function of aging?
McGeer EG. Neurotransmitter systems in aging and senile dementia.
McIntosh AR, Sekuler AB, Penpeci C, Rajah MN, Grady CL, Sekuler R, et al. Recruitment of unique neural systems to support visual memory in normal aging.
Mesulam MM. The human fronal lobes: transcending the default mode through contingent encoding. In: Stuss D, Knight RT, editors. Principles of frontal lobe function. 1st ed. New York: Oxford University Press;
Miguez JM, Aldegunde M, Paz-Valinas L, Recio J, Sanchez-Barcelo E. Selective changes in the contents of noradrenaline, dopamine and serotonin in rat brain areas during aging.
Milner B, Petrides M. Behavioural effects of frontal lobe lesions in man.
Mishkin M. Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus.
Mishkin M, Manning FJ. Non-spatial memory after selective prefrontal lesions in monkeys.
Mitchell KJ, Johnson MK, Raye CL, D'Esposito M. fMRI evidence of age-related hippocampal dysfunction in feature binding in working memory.
Mohr C, Landis T, Bracha HS, Brugger P. Opposite turning behaviour in right-handers and non-right-handers suggests a link between handedness and cerebral dopamine asymmetries.
Morcom AM, Good CD, Frackowiak RS, Rugg MD. Age effects on the neural correlates of successful memory encoding.
Morecraft RJ, Geula C, Mesulam MM. Architecture of connectivity within a cingulo-fronto-parietal neurocognitive network for directed attention.
Morgan DG. The dopamine and serotonin systems during aging in human and rodent brain. A brief review.
Moscovitch M, Winocur G. The frontal cortex and working with memory. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York: Oxford University Press;
Nielsen-Bohlman L, Knight RT. Prefrontal alterations during memory processing in aging.
Nowak G. Lateralization of neocortical dopamine receptors and dopamine level in normal Wistar rats.
Nyberg L, Marklund P, Persson J, Cabeza R, Forkstam C, Petersson KM, et al. Common prefrontal activations during working memory, episodic memory, and semantic memory.
O'Reilly RC, Noelle DC, Braver TS, Cohen JD. Prefrontal cortex and dynamic categorization tasks: representational organization and neuromodulatory control.
Oke A, Keller R, Mefford I, Adams RN. Lateralization of norepinephrine in human thalamus.
Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans.
Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex.
Orbelo DM, Testa JA, Ross ED. Age-related impairments in comprehending affective prosody with comparison to brain-damaged subjects.
Osiewacz HD, Hamann A. DNA reorganization and biological aging. A review.
Pandya DN, Yeterian EH. Architecture and connections of cortical association areas. In: Jones EG, Peters A, editors. Cerebral cortex. Vol. 4, New York: Plenum Press;
Park DC, Welsh RC, Marshuetz C, Gutchess AH, Mikels J, Polk TA, et al. Working memory for complex scenes: age differences in frontal and hippocampal activations.
Park DC, Polk TA, Park R, Minnear M, Savage A, Smith M. Aging reduces neural specialization in ventral visual cortex.
Passingham RE. Visual discrimination learning after selective prefrontal ablations in monkeys (Macaca mulatta).
Passingham RE. Delayed matching after selective prefrontal lesions in monkeys (Macaca mulatta).
Peters A. Structural changes in the normally aging cerebral cortex of primates.
Peters A, Sethares C, Moss MB. The effects of aging on layer 1 in area 46 of prefrontal cortex in the rhesus monkey.
Petrides M. Deficits in associative-learning tasks after frontal- and temporal-lobe lesions in man.
Petrides M. Specialized systems for the processing of mnemonic information within the primate frontal cortex.
Petrides M. Frontal lobes and working memory: Evidence from investigations of the effects of cortical excisions in nonhuman primates. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. Amsterdam: Elsevier 1994; 59–82.
Petrides M. Dissociable roles of mid-dorsolateral prefrontal and anterior inferotemporal cortex in visual working memory.
Petrides M, Pandya DN. Comparative architectonic analysis of the human and the macaque frontal cortex. In: Boller F, Grafman J, editors. Handbook of neuropsychology. Amsterdam: Elsevier;
Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns.
Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey.
Petrides M, Alivisatos B, Frey S. Differential activation of the human orbital, mid-ventrolateral, and mid-dorsolateral prefrontal cortex during the processing of visual stimuli.
Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging.
Ranganath C, Paller KA. Frontal brain activity during episodic and semantic retrieval: insights from event-related potentials.
Ranganath C, Johnson MK, D'Esposito M. Left anterior prefrontal activation increases with demands to recall specific perceptual information.
Raz N. Aging of the brain and its impact on cognitive performance: intergration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 2nd ed. Mahwah, New Jersey: Lawrence Erlbaum Associates;
Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, et al. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter.
Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume.
Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, et al. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET.
Riekkinen M, Aroviita L, Kivipelto M, Taskila K, Riekkinen P Jr. Depletion of serotonin, dopamine and noradrenaline in aged rats decreases the therapeutic effect of nicotine, but not of tetrahydroaminoacridine.
Robbins TW. Chemical neuromodulation of frontal-executive functions in humans and other animals.
Roberts NA, Beer JS, Werner KH, Scabini D, Levens SM, Knight RT, Levenson RW. The impact of orbital prefrontal cortex damage on emotional activation to unanticipated and anticipated acoustic startle stimuli.
Rodriguez M, Martin L, Santana C. Ontogenic development of brain asymmetry in dopaminergic neurons.
Rosen AC, Prull MW, O'Hara R, Race EA, Desmond JE, Glover GH, et al. Variable effects of aging on frontal lobe contributions to memory.
Rugg MD, Otten LJ, Henson RN. The neural basis of episodic memory: evidence from functional neuroimaging.
Rugg MD, Henson RN, Robb WG. Neural correlates of retrieval processing in the prefrontal cortex during recognition and exclusion tasks.
Rypma B, D'Esposito M. Isolating the neural mechanisms of age-related changes in human working memory.
Rypma B, Prabhakaran V, Desmond JE, Gabrieli JD. Age differences in prefrontal cortical activity in working memory.
Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E., et al. Thinning of the cerebral cortex in aging.
Schiavetto A, Kohler S, Grady CL, Winocur G, Moscovitch M. Neural correlates of memory for object identity and object location: effects of aging.
Schreursa BG, Bahro M, Molchan SE, Sunderland T, McIntosh AR. Interactions of prefrontal cortex during eyeblink conditioning as a function of age.
Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex.
Shimamura AP. Memory and frontal lobe function. In: Gazzaniga MS, editor. The cognitive neurosciences. Cambridge, MA: MIT Press;
Slopsema JS, van der Gugten J, de Bruin JP. Regional concentrations of noradrenaline and dopamine in the frontal cortex of the rat: dopaminergic innervation of the prefrontal subareas and lateralization of prefrontal dopamine.
Smith EE, Geva A, Jonides J, Miller A, Reuter-Lorenz P, Koeppe RA. The neural basis of task-switching in working memory: effects of performance and aging.
Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life.
Stefanacci L, Amaral DG. Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study.
Stuss DT, Alexander MP. Executive functions and the frontal lobes: a conceptual view.
Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York City, NY: Oxford University Press;
Stuss DT, Floden D, Alexander MP, Levine B, Katz D. Stroop performance in focal lesion patients: dissociation of processes and frontal lobe lesion location.
Stuss DT, Alexander MP, Floden D, Binns MA, Levine B, McIntosh AR, et al. Fractionation and localization of distinct frontal lobe processes: evidence from focal lesions in humans. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York City, NY: Oxford University Press;
Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers Inc.;
Taoka T, Iwasaki S, Uchida H, Fukusumi A, Nakagawa H, Kichikawa K, et al. Age correlation of the time lag in signal change on EPI-fMRI.
Tisserand DJ, Jolles J. On the involvement of prefrontal networks in cognitive ageing.
Tisserand DJ, Pruessner JC, Sanz Arigita EJ, van Boxtel MP, Evans AC, Jolles J, et al. Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel-based morphometry.
Tisserand DJ, Van Boxtel MP, Pruessner JC, Hofman P, Evans AC, Jolles J. A voxel-based morphometric study to determine individual differences in gray matter density associated with age and cognitive change over time.
Trott CT, Friedman D, Ritter W, Fabiani M. Item and source memory: differential age effects revealed by event-related potentials.
Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of visual behaviour. Cambridge: MIT Press;
Ungerleider LG, Gaffan D, Pelak VS. Projections from inferior temporal cortex to prefrontal cortex via the uncinate fascicle in rhesus monkeys.
Uylings HB, de Brabander JM. Neuronal changes in normal human aging and Alzheimer's disease.
van Dyck CH, Seibyl JP, Malison RT, Laruelle M, Wallace E, Zoghbi SS, et al. Age-related decline in striatal dopamine transporter binding with iodine-123-beta-CITSPECT.
van Dyck CH, Seibyl JP, Malison RT, Laruelle M, Zoghbi SS, Baldwin RM, et al. Age-related decline in dopamine transporters: analysis of striatal subregions, nonlinear effects, and hemispheric asymmetries.
Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals.
Volkow ND, Wang GJ, Fowler JS, Ding YS, Gur RC, Gatley J, et al. Parallel loss of presynaptic and postsynaptic dopamine markers in normal aging.
Volkow ND, Logan J, Fowler JS, Wang GJ, Gur RC, Wong C, et al. Association between age-related decline in brain dopamine activity and impairment in frontal and cingulate metabolism.
Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis.
Wagner AD, Maril A, Bjork RA, Schacter DL. Prefrontal contributions to executive control: fMRI evidence for functional distinctions within lateral prefrontal cortex.
Walker AE. A cytoarchitectural study of the prefrontal area of the macaque monkey.
Watkins KE, Paus T, Lerch JP, Zijdenbos A, Collins DL, Neelin P, et al. Structural asymmetries in the human brain: a voxel-based statistical analysis of 142 MRI scans.
Wegesin DJ, Friedman D, Varughese N, Stern Y. Age-related changes in source memory retrieval: an ERP replication and extension.
West R. In defense of the frontal lobe hypothesis of cognitive aging.