-
PDF
- Split View
-
Views
-
Cite
Cite
Raquel Dorsaint-Pierre, Virginia B. Penhune, Kate E. Watkins, Peter Neelin, Jason P. Lerch, Marc Bouffard, Robert J. Zatorre, Asymmetries of the planum temporale and Heschl's gyrus: relationship to language lateralization, Brain, Volume 129, Issue 5, May 2006, Pages 1164–1176, https://doi.org/10.1093/brain/awl055
Close - Share Icon Share
Abstract
Morphological asymmetries favouring the left hemisphere in the planum temporale (PT) and Heschl's gyrus (HG) have both been presumed to relate to the typical left-hemisphere dominance for language functions. However, a direct link between structure and function has not been clearly established. The present study investigates this issue by measuring the volume of the PT and HG on the MRI scans of epilepsy patients classified into three groups: left speech group (LSG; n = 20), right speech group (RSG; n = 11) and bilateral speech group (BSG; n = 13), as assessed by the intracarotid Sodium Amytal procedure. Additionally, an automatic voxel-based morphometry (VBM) analysis was performed to explore collateral structural asymmetries. Although leftward structural asymmetries were found in the PT, consistent with the literature, they did not relate to language lateralization. For HG we also replicated asymmetries favouring the left side; interestingly, three of the individuals within the RSG showed a strongly reversed asymmetry, but as a whole the structure–function relationship for HG was not obligatory. The VBM analysis revealed a grey-matter concentration difference in the posterior part of the inferior frontal gyrus (pars opercularis, corresponding functionally to Broca's area), which favoured the left hemisphere in the LSG, and the right hemisphere in the RSG. The findings suggest that this frontal cortical region bears a direct relationship to language lateralization, which may be related to use-dependent plasticity in patients with language reorganization.
Introduction
The functional specialization of the left cerebral hemisphere for language functions in most individuals has received ample confirmation. There is also much neuroanatomical evidence suggesting that structural differences between the two hemispheres exist. However, a clear structure–function relationship remains unproven. The present study was conducted to investigate this question systematically. More specifically, the relationship between structural asymmetries in the auditory cortices, the planum temporale (PT) and Heschl's gyrus (HG), and language lateralization was examined in epilepsy patients with known left- or right-hemisphere language lateralization as determined via intracarotid Sodium Amytal testing.
In their original study, Geschwind and Levitsky (1968) reported that the PT, a region found on the superior temporal plane and part of the classical Wernicke's area, was larger in size on the left hemisphere relative to the right. This finding was interpreted as providing the first clear neuroanatomical evidence for the left hemisphere's specialization for speech. Many studies have since then confirmed the PT asymmetry, which was found to be present pre- and peri-natally (e.g. Witelson and Pallie, 1973; Wada et al., 1975; Chi et al., 1977), strengthening the hypothesis that language lateralization is innately determined.
Although most researchers have agreed on the presence of an asymmetry in the PT, the nature of that asymmetry is controversial. One problem was that the incidence of left-hemisphere PT asymmetry (about 60–80%) was always much lower than the incidence of left-hemisphere language lateralization in the population (estimated to be 96% or higher). Another controversy relates to the nature of the measurement. Originally, Geschwind and Levitsky's post-mortem study, as well as others (e.g. Witelson and Pallie, 1973; Wada et al., 1975) had interpreted the PT asymmetry as one related to size, whereas other studies demonstrated that the asymmetry was related to shape or angulation (e.g. Rubens et al., 1976; Loftus et al., 1993; Binder et al., 1996; Westbury et al., 1999). One reason for this discrepancy is the lack of a clear consensus of what constitutes the borders of the PT (Shapleske et al., 1999; Westbury et al., 1999), particularly the posterior border. The use of the so-called knife-cut method has contributed to this problem.
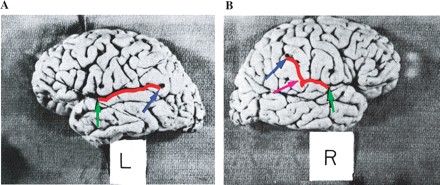
According to Rubens et al. (1976), previous researchers excluded cortical areas above the bottom end-point of the posterior ascending ramus from PT measurements (see Fig. 1B), believing that these areas pertained not to the PT, but rather to a region named planum parietale (PP) (Binder et al., 1996). Many subsequent researchers have, however, argued that there is little evidence to support the claim that the PT and PP are cytoarchitectonically (Witelson et al., 1995) or functionally different (e.g. Steinmetz et al., 1989; Binder et al., 1996), thus making the distinction a spurious one. Furthermore, several studies have clearly demonstrated that when the PT and PP are combined, a shape rather than a size asymmetry is observable (Rubens et al., 1976; Loftus et al., 1993; Binder et al., 1996; Honeycutt et al., 2000). Whereas the horizontal portion of the left PT is found to be longer than the right PT (see Fig. 1A), the right PT curves more anteriorly and extends vertically much more than the left PT (see Fig. 1B). According to many authors (e.g. Rubens et al., 1976; Loftus et al., 1993; Binder et al., 1996; Westbury et al., 1999) the PT size asymmetry observed by previous investigators is more of a by-product ‘of the more anterior upswing of the sylvian fissure [and the PT] on the right rather than a manifestation of functionally significant increase in size on the left’ (Loftus et al., 1993, p. 354). Thus, on the basis of this conclusion, the present study will measure the PT using both the knife-cut method and the combined PT and PP method (Westbury method) (Westbury et al., 1999), and will consider size as well as shape differences.
(A) Illustration of the left PT. Sagittal view of a post-mortem brain. The horizontal portion (from the green to the blue arrow) of the left PT is found to be longer than the right PT (seen in B from the green to the pink arrow). (B) Illustration of the right PT. Sagittal view of a post-mortem brain. The pink arrow points out the posterior ending of the horizontal portion (also referred to as the bottom end-point of the posterior ascending ramus) of the SF and PT, whereas the blue arrow points to the actual ending of the PT. The region between the pink and blue arrows has been traditionally excluded from PT measurements, as this region was believed to pertain to the PP or the posterior ascending ramus. Overall the right PT (from the green to the blue arrow) curves more anteriorly and extends more vertically than the left PT (seen in A) Original illustrations A and B reproduced with permission from Rubens AB, Mahowald MW, Hutton JT. Asymmetry of the lateral (sylvian) fissures in man. Neurology 1976; 26: 620–4.
Another structural asymmetry of interest is found in HG (e.g. von Economo and Horn, 1930; Rademacher et al., 1993; Kulynych et al., 1994; Penhune et al., 1996; Penhune et al., 2003), which contains primary auditory cortex (PAC) (Rademacher et al., 1993, 2001). In an MR-based study, Penhune et al. (1996) found that the left HG was associated with a larger white matter volume than was the right HG. It was hypothesized that this white-matter asymmetry was related to more efficient processing of rapidly changing temporal information, which is relevant for speech (Tallal et al., 1993; Zatorre et al., 2002). Other asymmetries have been found at the cellular level (Seldon, 1981a, b, 1982; Hutsler and Gazzaniga, 1996). Several anatomical and MR-based studies have reported a more widespread leftward asymmetry of white matter in posterior temporal areas (Anderson et al., 1999), and in frontal, parietal and temporal regions (Pujol et al., 2002). Thus, the present study will also examine the possible relationship between the white-matter asymmetry in HG and hemispheric specialization of speech functions.
The few studies that have attempted to investigate the relationship between structural and functional asymmetries have yielded mixed results (e.g. Steinmetz et al., 1991; Jäncke and Steinmetz, 1993; Foundas et al., 1994; Tzourio et al., 1998; Josse et al., 2003; Dos Santos Sequeira et al., 2006; Eckert et al., 2006; also see Witelson and Kigar, 1988, for a review). A number of investigators (e.g. Di Chiro, 1962; McRae et al., 1968; Ratcliff et al., 1980; Strauss et al., 1985) have tried to describe the relationship between morphological asymmetries and speech lateralization as determined via the intracarotid Amytal procedure (IAP), implemented to determine language lateralization in epilepsy patients (Branch et al., 1964). Of most direct relevance, Ratcliff et al. (1980) measured the angulation of the posterior branch of the middle cerebral artery from the angiograms of epilepsy patients who had undergone the IAP. This angle was compared between the two hemispheres serving as an index of the sylvian fissure (SF) shape asymmetry. Ratcliff et al.'s main findings were that patients who had left-hemisphere speech showed an asymmetry of the measured angle between the two hemispheres. Those who had bilateral or right-hemisphere speech showed a reduced asymmetry between the two hemispheres.
Foundas et al. (1994) were among the first to attempt to relate the PT asymmetry directly to speech lateralization, measured via the IAP. Using MR morphometry to measure PT length, they found that when speech was lateralized to the left hemisphere, the left PT was longer than the right PT, and, conversely, when speech was lateralized to the right hemisphere, the right PT was longer than the left PT. Unfortunately, only one subject was included in the right-hemisphere speech group, making it difficult to conclude whether these results would generalize to a larger sample of right speech individuals. Taken together, the existing data provide some support for the structure–function link; however, the evidence remains scarce and unconvincing.
The purpose of the present study was to investigate more systematically the morphological asymmetries in HG and PT, via MR analysis, in a series of epilepsy patients with known language lateralization as determined from the IAP, considered to be the most valid method for identifying hemispheric dominance (Perria et al., 1961; Branch et al., 1964; Risse et al., 1997). The following dissociation was predicted: (i) individuals with speech represented in the left hemisphere would show a leftward volume asymmetry in both HG and PT (measured via the knife-cut method), as well as a right–left angulation difference for the PT (measured via the Westbury method); and (ii) individuals with speech represented in the right hemisphere or bilaterally would show a reduced structural asymmetry in both structures (Ratcliff et al., 1980). To test this prediction, we examined three groups of patients with speech represented in the left hemisphere (left speech group: LSG), the right hemisphere (right speech group: RSG), or bilaterally (bilateral speech group: BSG).
Two different but complementary methodologies were used to investigate the structural asymmetries. The first involved manual labelling and volume measurement of the individual structures using three-dimensional computerized MR (Penhune et al., 1996; Westbury et al., 1999). The second, voxel-based morphometry (VBM) (Ashburner and Friston, 2000), is an exploratory whole-brain analysis that has the advantage of being reproducible and automated. This technique measures the distribution of grey matter and white matter, thus giving a comprehensive assessment of anatomical differences throughout the brain. The goal of using the automatic procedure was not only to confirm the manual labelling results in auditory regions but also to explore structural asymmetries elsewhere in the brain that could possibly relate to language lateralization.
Methods
Subjects
The subjects who participated in the present study were patients who suffered from intractable epilepsy, and who had come to the Montreal Neurological Institute (MNI) for surgery. The subjects gave their consent, according to the Declaration of Helsinki and through the applicable ethical committees of the MNI, to undergo both the IAP and MRI scanning, both procedures being part of their pre-surgical work-up. Demographic and clinical details of these patients are given in Table 1.
Clinical and demographic information for the three speech groups: LSG, RSG and BSG
| Group . | Age mean (range) . | Full-scale IQ mean (range) . | Handedness . | . | . | Gender . | . | Seizure onset (age range) . | Seizure focus . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | R . | L . | MIX . | M . | F . | . | L . | R . | BI . | N/A . | ||||||
| LSG (n = 20) | 37 (20–61) | 89 (72–112) | 15 | 4 | 1 | 9 | 11 | 1–50 years | 5 | 9 | 1 | 5 | ||||||
| RSG (N = 11) | 36 (10–54) | 81 (67–98) | 4 | 6 | 1 | 4 | 7 | 1 month–25 years | 8 | 0 | 1 | 2 | ||||||
| BSG (N = 13) | 35 (11–48) | 84 (67–100) | 5 | 8 | 0 | 6 | 7 | Birth–25 years | 7 | 2 | 2 | 2 | ||||||
| Group . | Age mean (range) . | Full-scale IQ mean (range) . | Handedness . | . | . | Gender . | . | Seizure onset (age range) . | Seizure focus . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | R . | L . | MIX . | M . | F . | . | L . | R . | BI . | N/A . | ||||||
| LSG (n = 20) | 37 (20–61) | 89 (72–112) | 15 | 4 | 1 | 9 | 11 | 1–50 years | 5 | 9 | 1 | 5 | ||||||
| RSG (N = 11) | 36 (10–54) | 81 (67–98) | 4 | 6 | 1 | 4 | 7 | 1 month–25 years | 8 | 0 | 1 | 2 | ||||||
| BSG (N = 13) | 35 (11–48) | 84 (67–100) | 5 | 8 | 0 | 6 | 7 | Birth–25 years | 7 | 2 | 2 | 2 | ||||||
IQ was defined from the WAIS-R; handedness was based on a standard questionnaire; seizure focus was determined from side of predominant EEG abnormality; BI = bilateral, N/A = could not be determined. LSG = left speech group; RSG = right speech group; BSG = bilateral speech group.
Clinical and demographic information for the three speech groups: LSG, RSG and BSG
| Group . | Age mean (range) . | Full-scale IQ mean (range) . | Handedness . | . | . | Gender . | . | Seizure onset (age range) . | Seizure focus . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | R . | L . | MIX . | M . | F . | . | L . | R . | BI . | N/A . | ||||||
| LSG (n = 20) | 37 (20–61) | 89 (72–112) | 15 | 4 | 1 | 9 | 11 | 1–50 years | 5 | 9 | 1 | 5 | ||||||
| RSG (N = 11) | 36 (10–54) | 81 (67–98) | 4 | 6 | 1 | 4 | 7 | 1 month–25 years | 8 | 0 | 1 | 2 | ||||||
| BSG (N = 13) | 35 (11–48) | 84 (67–100) | 5 | 8 | 0 | 6 | 7 | Birth–25 years | 7 | 2 | 2 | 2 | ||||||
| Group . | Age mean (range) . | Full-scale IQ mean (range) . | Handedness . | . | . | Gender . | . | Seizure onset (age range) . | Seizure focus . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | R . | L . | MIX . | M . | F . | . | L . | R . | BI . | N/A . | ||||||
| LSG (n = 20) | 37 (20–61) | 89 (72–112) | 15 | 4 | 1 | 9 | 11 | 1–50 years | 5 | 9 | 1 | 5 | ||||||
| RSG (N = 11) | 36 (10–54) | 81 (67–98) | 4 | 6 | 1 | 4 | 7 | 1 month–25 years | 8 | 0 | 1 | 2 | ||||||
| BSG (N = 13) | 35 (11–48) | 84 (67–100) | 5 | 8 | 0 | 6 | 7 | Birth–25 years | 7 | 2 | 2 | 2 | ||||||
IQ was defined from the WAIS-R; handedness was based on a standard questionnaire; seizure focus was determined from side of predominant EEG abnormality; BI = bilateral, N/A = could not be determined. LSG = left speech group; RSG = right speech group; BSG = bilateral speech group.
The intracarotid Amytal procedure (IAP)
The IAP procedure consists in anaesthetizing one hemisphere at a time using sodium amobarbital (Amytal) and evaluating the speech potential of the contralateral ‘awake' hemisphere. Each hemisphere is tested on a separate day. After being tested on both sides, the patient is classified as having speech represented in the left hemisphere, the right hemisphere or bilaterally according to the criteria that include the presence of speech arrest and variable degrees of errors on speech tasks (e.g. naming, serial speech, comprehension, reading, spelling) when the dominant hemisphere is injected, and the absence of these speech difficulties when the opposite hemisphere is injected (Perria et al., 1961; Branch et al., 1964; Zatorre, 1989; Jones-Gotman, 1997). In cases where speech is represented bilaterally, a few possible scenarios can be observed, ranging from the patient showing no apparent speech disturbances from injection of either hemisphere to the patient showing considerable speech disturbances after injection of both hemispheres. In this study all bilateral speech cases were merged to form a single speech group (BSG). On the basis of the IAP's criteria mentioned above, 20 patients were classified as having speech functions in the left hemisphere (LSG), 11 patients were classified as having speech functions in the right hemisphere (RSG) and 13 patients were classified as having speech represented bilaterally (BSG).
MR scanning
A total of 47 patients were initially selected for the present study on the basis of availability of adequate MR scans and data on the IAP; however, 3 were excluded because they were found to have structural lesions or tumours located in proximity to the speech areas of interest; the remaining subjects were free of gross structural abnormalities. Pre-operative scans were used in 42 cases. In two cases only post-operative scans were available. These patients had undergone a selective amygdalo-hippocampectomy with some resection in the anterior temporal lobe; however, they were included in the study as they did not reveal any important lesions near the temporal speech regions of interest. Thus, 44 patients were included in the volumetric analysis. For the VBM analysis one additional patient's scan was excluded (from the RSG) because it was of insufficient technical quality.
Volumetry analysis
MR image acquisition and analysis
All but one MRI scans were obtained on a Phillips Gyroscan system with a 1.5 T superconducting magnet. The remaining MRI scan was obtained on a Siemens 1.5 T system. Three-dimensional fast field echo T1-weighted images [with TR (repetition time) range = 18–27 ms, TE (echo time) range = 6–10 ms and the flip angle range = 30–45°, with 145–180 contiguous sagittal slices and 1 × 1 × 1 mm3 voxels] were acquired. The MR images were transformed into standardized MNI stereotaxic space (Talairach and Tournoux, 1988) using an automatic nine-parameter linear algorithm (Collins et al., 1994) based on the MNI 305 target. This procedure systematizes the orientation of orthogonal planes of section and permits control over overall brain-size differences, while also allowing for direct voxel-by-voxel comparisons across individual scans.
Operational identification of the borders of HG and the PT
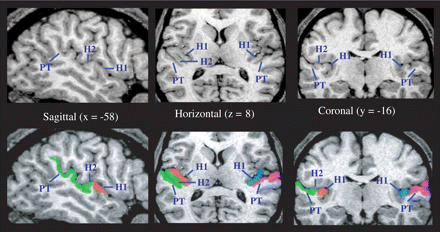
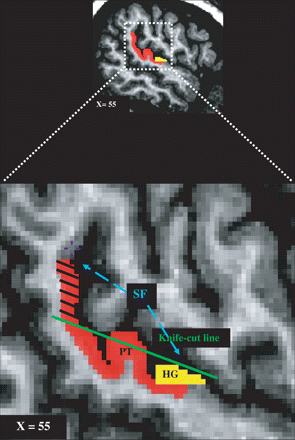
HG and the PT were identified and labelled using methods and criteria developed by Penhune et al. (1996) and Westbury et al. (1999) respectively (see Fig. 2). For the PT, volume was measured two ways: including all tissue pertaining to the temporal plane (Westbury method), as well as using the more conventional knife-cut method, to allow better comparison of the results with those of earlier ones (see Fig. 3).
Identification and labelling of HG and PT. If two HGi were observed, the second HG was included as part of the PT. H1 = first Heschl's gyrus; H2 = second Heschl's gyrus; PT = planum temporale.
Illustration of how the knife-cut method was implemented on an MRI scan. Sagittal view of the labelled volume in the right hemisphere. A projection line (knife-cut line) mimicking the knife trajectory along the SF was imagined and the labels falling above that line (blue dash lines) were removed. PT = planum temporale; HG = Heschl's gyrus; SF = sylvian fissure.
Procedure and analysis
HG and PT were manually labelled on all MR scans using DISPLAY software (MacDonald et al., 1994). Both HG and PT were labelled by a single rater (R.D.P.) who was blind to both speech group and hemisphere (half the scans were randomly flipped across the x-axis to ensure no bias in labelling of each hemisphere). Only grey-matter voxels were labelled for the PT, while both grey and white-matter voxels were labelled for HG. Volumes of labelled structures were calculated by summing all labelled voxels. For HG, grey and white matter were segmented using an automatic tissue classification algorithm, which operates on an artificial neural network classifier (Zijdenbos et al., 1996). Additionally for the PT, angulation slope, an index of shape, was computed by fitting the labelled structure into a three-dimensional bounding box, from which the minimum and maximum x, y, z coordinates were extracted (see Westbury et al., 1999, for further details). The slope was computed by taking the height-over-length ratio.
VBM analysis
The second technique used to complement the manual labelling analysis was VBM (Ashburner and Friston, 2000). This methodology is described in greater detail elsewhere (see Paus et al., 1999; Ashburner and Friston, 2000; Good et al., 2001; Watkins et al., 2001). After stereotaxic normalization and tissue classification, the binary grey-matter maps were smoothed using a Gaussian smoothing kernel of 10-mm full-width at half-maximum, resulting in three-dimensional maps of tissue concentration. We used VBM to examine interhemispheric structural asymmetries across the whole brain on a voxel-wise level; we therefore adopted a symmetrical template as the target to which all individual volumes were registered. Using an asymmetric (i.e. standard) template could enhance the asymmetries of the original data set (Watkins et al., 2001), but the symmetrical template bypasses this problem. To investigate interhemispheric asymmetries, we created image scans representing the differences in the amounts of grey matter between left and right hemispheres; thus the images were flipped across the midline (x = 0) and subtracted from the unflipped images. A positive voxel value on the left side of the final image corresponded to a higher signal (i.e. more grey-matter concentration) on the left hemisphere than on the right, and vice-versa; these final difference image scans were then statistically analysed at each voxel producing t-statistic maps, following a similar procedure to that used for functional data (Worsley et al., 1996).
Two sets of analyses were performed. For the first analysis, the difference maps were averaged across all three speech groups, to look at the overall grey-matter concentration differences between the two hemispheres irrespective of group. To correct for multiple comparisons involved in searching across a brain volume a threshold of t > 5.5, a P-significance level <0.05, 42 degrees of freedom (df), a voxel size equal to 1 mm3, a smoothing kernel of 10 mm and a standard volume of interest of 1000 cc were used (Worsley et al., 1996). For the second analysis, the difference maps of the two unilateral speech groups, RSG and LSG, were contrasted with one another. We believed that any possible interhemispheric grey-matter differences that existed would be best revealed by examining these two particular speech groups since they demonstrated opposite functional lateralization patterns; a threshold of t > 6.1 and df = 28 were used.
Results
Volumetry analysis
Heschl's gyrus
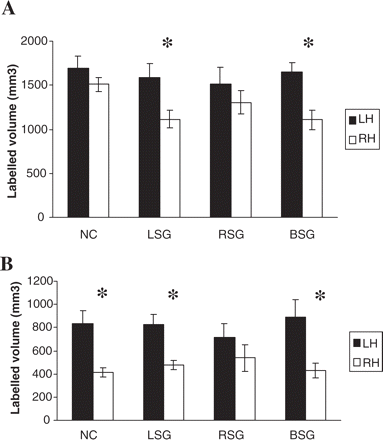
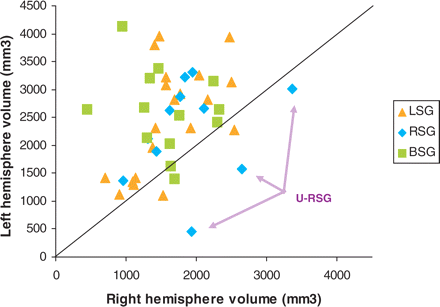
A repeated-measures ANOVA (analysis of variance) was performed on the segmented grey- and white-matter HG labelled volumes. The speech group (LSG/RSG/BSG) was the between-subjects variable, and the hemisphere and tissue type were the within-subjects variables. The analysis revealed an overall leftward asymmetry that was significant for both tissue types, Fgrey(1, 41) = 23.50, P < 0.0001; Fwhite(1, 41) = 24.16, P < 0.0001. Although there was no group × hemisphere interaction, planned comparisons showed an asymmetry favouring the left for both the LSG [Fgrey(1,41) = 15.22, P < 0.001; Fwhite(1,41) = 13.33, P < 0.001] and the BSG [Fgrey(1,41) = 13.24, P < 0.001; Fwhite(1,41) = 14.84, P < 0.001] but not the RSG [Fgrey (1,41) = 1.60, P > 0.05; Fwhite(1,41) = 1.84, P > 0.05]. These results are consistent with those of Penhune et al. (1996) performed on normal right-handed control subjects (presumably equivalent to the LSG in this study). Importantly, the volumes of HG in the patient population in the present study were within a comparable range as those of Penhune et al.'s normal control subjects (see Fig. 4), and did not differ significantly (Pgrey = 0.34; Pwhite = 0.98) from them. A scatter plot displaying total left and right HG volume for all individuals within each of the three groups showed that three RSG subjects had a pronounced right larger than left volume difference, larger than that for any of the other subjects (see Fig. 5). These results contributed to the lack of overall L > R volume difference in the RSG.
Mean interhemispheric volume difference (±SE) measured for HG for the three speech groups compared with a normal control group from the study of Penhune et al. (1996). (A) Grey-matter volumes. (B) White-matter volumes. NC = normal control group; LSG = left speech group; RSG = right speech group; BSG = bilateral speech group; LH = left hemisphere; RH = right hemisphere. The asterisk (*) = significant interhemispheric differences, P < 0.0001.
Scatterplot displaying the labelled volumes for HG (grey and white matter summed together) for the three speech groups, showing in particular the three RSG subjects (unusual-RSG: U-RSG) who had a more pronounced right larger than left volume difference.
Planum temporale
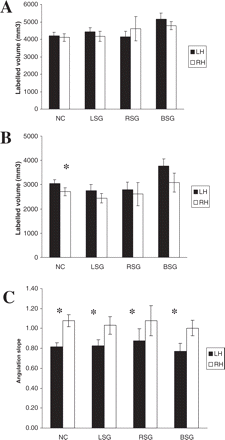
A repeated-measures ANOVA was performed on the PT volumes labelled using the Westbury method. Speech group was the between-subjects variable, and hemisphere was the within-subjects variable. The analysis showed no significant interhemispheric differences in PT volume across the three speech groups, F(1,41) = 0.06, P > 0.05. There was also no group × hemisphere interaction. Westbury et al., (1999) found similar results for a normal control group of subjects (equivalent to the LSG in this study). Again, the patients' PT volumes were similar to the volumes of Westbury et al.'s normal control subjects (see Fig. 6A). A total of 22 subjects showed an L > R asymmetry and likewise 22 subjects showed an R > L asymmetry.
A similar analysis performed after the knife-cut method was applied revealed a marginally significant interhemispheric difference in PT volume across all three groups, F(1,41) = 3.02, P = 0.09. Again there was no group × hemisphere interaction. Previously, Westbury et al., (1999) did show a significant interhemispheric difference for normal control subjects using this method; here a similar trend was apparent (see Fig. 6B). A total of 27 subjects showed an L > R asymmetry, of whom 11 were from the LSG, whereas 17 subjects showed an R > L asymmetry, of whom nine were from the LSG.
Mean interhemispheric volume difference (±SE) measured for the PT. (A) Westbury method. (B) Knife-cut method. (C) Mean interhemispheric angulation difference (±SE) measured for the PT. All comparisons are between the three speech groups and a normal control group from the study of Westbury et al. (1999). In B the asterisk (*) = significant interhemispheric differences, P < 0.05. In C the asterisk (*) = significant interhemispheric differences, P < 0.05 (for NC) and 0.01 (for LSG, RSG and BSG). Abbreviations as in Fig. 4.
Finally, the ANOVA for the PT angulation measure revealed a significant interhemispheric difference such that the right side had a steeper slope across all three groups, F(1,41) = 7.8, P < 0.01, confirming the findings of Westbury et al. (1999), for normal control subjects (see Fig. 6C). A total of 31 subjects showed an R > L asymmetry whereas 13 subjects showed an L > R asymmetry. No group × hemisphere interaction was found.
VBM analysis
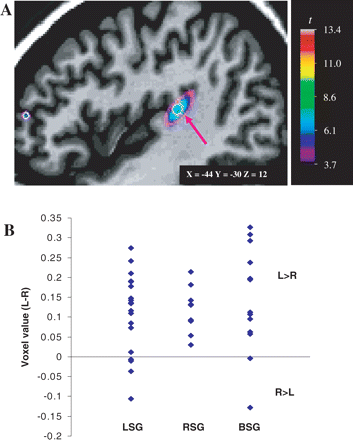
In the first VBM analysis the hemispheric difference maps of the subjects across all three groups were averaged, yielding the mean interhemispheric difference in grey-matter concentration. Some of the most significant peaks obtained were located in regions comparable with those previously discussed in Watkins et al. (2001) (see Tables 2 and 3 in supplementary section at Brain online). These include the right frontal (x = 10, y = 61, z = 25) and left occipital (x = −15, y = −86, z = 37) petalia, the right superior temporal sulcus (x = 48, y = −23, z = −6 ) and the caudate nucleus (x = 15, y = 15, z = 17). Of greatest interest is the PT result, which showed that there was more grey matter on average in the left hemisphere compared with the right one in this region; the statistical peak found at coordinates x = −44, y = −30, z = 12 (corresponding to BA 42 and/or BA 22) was significant, t = 7.91, P < 0.05 (see Fig. 7A). The voxel value at that peak's coordinates was then extracted from the average map for each subject across the three groups and these values were analysed to better investigate the group distributions. All three speech groups showed a similar distribution pattern (see Fig. 7B), with most subjects showing a positive voxel value, indicative of more grey matter on the left side than on the right. There were no significant group differences found on a one-way ANOVA, F(2,40) = 0.35, P > 0.05. Thus, the voxel-based analysis was consistent with the manual PT labelling results in showing an overall asymmetry that was identical across all three speech groups.
(A) Sagittal view of the left hemisphere. Voxel-based morphometric statistical map averaged across the three speech groups superimposed on the MRI scan of a single subject. A statistical peak in the region of the PT is shown here (arrow), which reveals an interhemispheric asymmetry in grey-matter concentration in the PT across all three speech groups. (B) Distribution of the voxel values extracted at the statistical peak corresponding to the Talairach coordinates of the PT (−44, −30, 12) for the three speech groups. L > R = more grey-matter concentration in the left hemisphere; R > L = more grey-matter concentration in the right hemisphere. Other abbreviations as in Fig. 4.
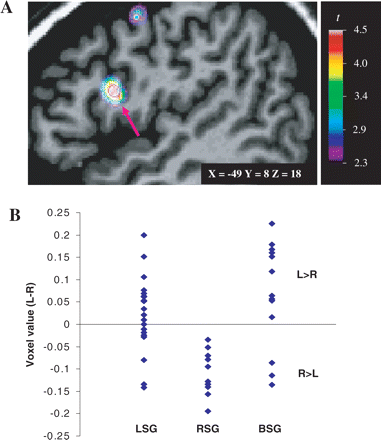
In the second VBM analysis, the maps of the LSG and the RSG were contrasted to give the mean interhemispheric difference map of grey-matter concentration between those two groups. An important finding was revealed in the left posterior–inferior frontal cortex, functionally corresponding to Broca's area. The result showed that at this one point there was more grey matter in the left hemisphere for the LSG, whereas there was more grey matter in the right hemisphere for the RSG. The statistical peak corresponding approximately to BA 44 (Tomaiuolo et al., 1999) (coordinates: x = −49, y = 8, z = 18; Fig. 8A) was the peak with the highest t-value in this analysis, although it did not reach conventional levels of significance correcting for multiple comparisons (t = 4.51, Pcorrected > 0.1; Puncorrected < 0.00001). The voxel values were again extracted at that peak's coordinates and plotted to show the group distributions. Most of the LSG subjects showed a positive voxel value, indicative of more grey matter in the left hemisphere, whereas every single RSG subject showed a negative voxel value, indicative of more grey matter in the right hemisphere (see Fig. 8B).
(A) Sagittal view of the left hemisphere. Voxel-based morphometric statistical difference map contrasting the two speech groups, LSG and RSG, superimposed on the MRI scan of a single subject. A statistical peak in the pars opercularis, functionally known as Broca's area, is shown here (arrow), and it reveals an interhemispheric asymmetry where LSG had more grey matter in the left hemisphere than RSG. (B) Distribution of the voxel values extracted at the statistical peak corresponding to the Talairach coordinates of the pars opercularis (−49, 8, 18) for the three speech groups. L > R = more grey-matter concentration in the left hemisphere; R > L = more grey-matter concentration in the right hemisphere. Other abbreviations as in Fig. 4.
A one-way ANOVA on these data, with speech group the between-subjects variable and voxel-value difference between left and right hemispheres the dependent variable, revealed a significant speech group difference, F(2,40) = 11.30, P < 0.001. A Tukey HSD post hoc pairwise comparison showed that the RSG was significantly different from both the LSG (mean difference = −0.13, P = 0.002) and the BSG (mean difference = −0.17, P < 0.001), but the LSG was not significantly different from the BSG (mean difference = −0.043, P = 0.38).
Discussion
Volumetry analysis
Heschl's gyrus
The volumetric results showed the expected asymmetries favouring the left hemisphere for both HG white matter and grey matter. More specifically, interhemispheric differences were found for the LSG and the BSG, but not for the RSG. This was largely due to three RSG cases that had a reverse asymmetry more pronounced than any other subjects. This finding brings up an important issue related to the RSG, which is whether these patients had right speech representation innately or due to an early injury to the left hemisphere that caused speech functions to reorganize to the right hemisphere.
Earlier studies suggested that for speech to shift in the case of early injury, the left-sided injuries need to have happened before approximately the age of five years old, and quite large areas within primary speech zones need to have suffered the damage (Branch et al., 1964; Rasmussen and Milner, 1977). However, more recent research modifies these claims. Studies on aphasic patients showed that following left hemisphere damage, language functions can reorganize to the right hemisphere (e.g. Cappa and Vallar, 1992; Cappa et al., 1997; Thulborn et al., 1999) even in older adults. Similarly, a functional MRI (fMRI) study by Liégeois et al. (2004) showed that speech did not necessarily reorganize to the right hemisphere when lesions, however extensive, affected the primary speech areas.
It could be hypothesized that the three unusual RSG cases suffered some degree of atrophy in the left HG, resulting in the right HG appearing larger. However, only one of the three RSG cases had a slightly smaller than usual left volume. The other two RSG cases were well within the normal range for both left and right volumes. Thus, on average, atrophy per se cannot explain the present findings. Conversely, it could be that these three individuals represent a native reversal of the typical structural asymmetry and that the others in the RSG who show the typical HG pattern have retained the usual structural asymmetry favouring the left hemisphere, while undergoing functional reorganization of language functions to the right hemisphere. It was difficult, on the basis of the patients' history to determine with any certainty whether the right speech cases were due to innate or due to pathological factors. The patients included in the analysis did not have gross or extended morphological anomalies and the epileptogenic tissue was most often localized in the hippocampal region relatively far from lateral cortical speech areas, arguing against a lesion-driven reorganization. We conclude that right speech representation appears to be a consequence of a complex process where genetic, pathological, and environmental factors may interact such that structural and functional asymmetries can be dissociated in certain cases.
Planum temporale
As expected, no interhemispheric PT volume differences were found when the Westbury method was implemented, but there was a clear PT angulation difference, such that the right PT was more steeply angled than the left, corroborating the findings of Westbury et al. (1999). In early papers (e.g. Geschwind and Levitsky, 1968; Witelson and Pallie, 1973) it was speculated that a relationship existed between PT size asymmetry and functional asymmetry. But some later researchers have argued against this original thought and have suggested that PT shape asymmetry would relate to functional asymmetry. The present findings bring more evidence against the early speculation by showing that, when the PP was included in the PT measurement, there were no PT size or volume asymmetries, even in the LSG. However, a relationship between PT shape or angulation asymmetry and functional asymmetry was expected but also failed to be observed; all three speech groups showed the same rightward PT angulation difference. Previously, interhemispheric asymmetry in PT volume was obtained when the knife-cut method was used, but this PT difference did not reach significance in the present study, although a trend in the right direction was evident. In any case, since all three speech groups showed the same apparent trend, it is safe to conclude that neither with nor without the knife-cut approach is the PT asymmetry related to language lateralization. Taken together, these new findings suggest that although PT morphological asymmetries are clearly evident, they appear to bear no direct relationship to language lateralization.
This conclusion is inconsistent with some prior studies that have observed a different pattern of structural asymmetry in the different speech groups. Ratcliff et al. (1980) found that the right and BSGs had a reduced angiographic asymmetry compared with the LSG, while Foundas et al. (1994) found a reversal of the PT asymmetry in one right speech patient compared with the left speech patients. However, Foundas et al. only had one right speech patient, and the present results suggest that when a larger sample is used, on average, the RSG shows a similar asymmetry pattern as does the LSG. Ratcliff et al.'s study had similar sample sizes to the present one, and thus had sufficient power, but they measured only the asymmetry in the SF indirectly via measuring the angle of the middle cerebral artery. Thus, it can be speculated that structural changes at the level of the artery are not necessarily and directly reflected in the SF and consequently in the PT.
The present findings are, however, consistent with more recent structural and functional studies that have forwarded the notion that PT asymmetry may not relate to language lateralization in any direct way (e.g. Binder et al., 1996; Tzourio et al., 1998; Josse et al., 2003; Dos Santos Sequeira et al., 2006; Eckert et al., 2006). Of direct relevance, two recent studies, one by Eckert et al. (2006) and the other by Dos Santos Sequeira et al. (2006), investigated whether the asymmetry of the PT related to language lateralization in normal individuals. Using an fMRI single-word comprehension task and a dichotic listening task, respectively, to measure language lateralization, both studies observed a leftward PT asymmetry, but neither of them found that it corresponded to language lateralization, reinforcing the present findings.
Griffiths and Warren (2002) have reviewed many functional imaging studies that have found activation in the PT relative to different types of sound-processing tasks, and these authors presented a model whereby the PT was conceptualized as a region involved more generally in analyzing complex spectrotemporal patterns. Thus, the PT may play a more general role in auditory processing rather than a specific role in language-related processes, and thus may not relate specifically to language lateralization as initially conceived.
VBM analysis
The goal behind using the VBM analysis in the present study was to (i) confirm the volumetric data and (ii) explore other possible structural asymmetries in the brain that could relate to language lateralization. Consistent with the volumetric measures, the VBM results revealed an interhemispheric difference favouring the left hemisphere in grey-matter concentration for the PT. Earlier VBM studies have also found a clear left–right asymmetry in the PT in normal subjects (Good et al., 2001; Watkins et al., 2001) and interpreted it as one related to interhemispheric volume difference. Since VBM detected an interhemispheric difference in the PT, and we know from the volumetric analysis that volumes were not significantly different across hemispheres, we are tempted to conclude that the grey-matter concentration differences seen in the VBM analysis are related to the shape differences observed between the PTs, rather than to a volume difference. Also, it should be mentioned that VBM failed to detect the grey- and white-matter volume differences found via the volumetric analysis in HG. Taken together, these findings suggest that, although VBM is sensitive to morphologically based differences between the two hemispheres, these differences need not be related solely to volume (see also Tisserand et al., 2004). Insofar as the VBM findings were similar across all three speech groups, we again conclude that interhemispheric PT differences, whether due to shape or not, do not relate directly to speech lateralization.
The more relevant finding obtained via VBM was an interhemispheric difference in grey-matter concentration in the posterior part of the inferior frontal gyrus (i.e. pars opercularis, corresponding functionally to Broca's area), between the LSG, who showed a greater left than right-hemisphere grey-matter concentration, and the RSG, who showed the opposite asymmetry. Since this was the statistical peak of highest significance in the present analysis, and given that this is a strongly predictable region, the interhemispheric difference in the pars opercularis can be clearly considered a reliable finding. Furthermore, the analysis at the voxel-value level showed that all the individuals within the RSG had more grey-matter concentration favouring the right hemisphere, whereas most LSG subjects had grey-matter concentration favouring the left hemisphere. These results suggest that the structural asymmetry in the pars opercularis may represent an important neuroanatomical substrate for language lateralization.
An important question is whether the grey-matter concentration difference observed in the pars opercularis is one related to volume or to shape differences, as discussed earlier for the PT-related findings. Tomaiuolo et al. (1999) found no significant interhemispheric differences in this region using volumetry, but other studies did observe a left–right asymmetry in this region, favouring the left hemisphere (Foundas et al., 1998; Amunts et al., 1999). Although these studies measured volume, they did not consider whether shape differences existed. Thus, from the present state of results, it is not possible to clearly attribute the observed grey-matter concentration differences in the present data as a difference in the shape or volume of the pars opercularis. Since these interhemispheric differences appear to bear some relationship to speech lateralization, further research of this region is definitely warranted.
Methodological issues
One important limiting factor in the present study is that measures were taken in epileptic patients who may have atypical brain anatomy due to atrophy or other factors. Thus, the present structural and VBM findings should be considered with caution. Nonetheless, given that two recent studies (Eckert et al., 2006; Dos Santos Sequeira et al., 2006) found similar structural results in normal individuals, it seems unlikely that the present results apply exclusively to the epilepsy population. Also, there does not appear to be any evidence for atrophy in the regions measured, since the distributions of volumes of the relevant structures were comparable with those of normal control samples from previous studies (Penhune et al., 1996; Westbury et al., 1999). Thus, it is unlikely that atrophy per se of the structures of interest played any role in the results. It should also be noted that these subjects' volumes were transformed into standardized stereotaxic space, whose main advantage is to correct for overall volume differences (see Westbury et al., 1999). Thus, the interhemispheric differences observed are related to the concerned region of interest and uncontaminated by possible total volume differences. Another methodological point is that the volumes were measured blind, such that no bias could have been introduced, a precaution not always taken in such studies.
Structure–function relationship related to speech processing
From the results based on the volumetric and VBM analyses, two regions have been found to bear at least some relationship with language lateralization: HG and Broca's area. Two hypotheses can be proposed to explain this possible structure–function relationship. For HG, it was seen that only three RSG subjects showed a right larger than left volume asymmetry; the other eight RSG individuals had the same left larger than right volume asymmetry as the LSG and the BSG subjects. This suggests that the anatomical asymmetry does not necessarily follow the direction of the functional asymmetry. In other words, the structure–function relationship is not obligatory in HG. The fact that deaf subjects also showed the expected left larger than right volume differences in the absence of auditory stimulation (Emmorey et al., 2003; Penhune et al., 2003) provides further evidence to suggest that morphological asymmetries in the auditory cortex regions may be predetermined at an early developmental stage. Nonetheless, these auditory regions may still interact to some extent with environmental input. For example, Schneider et al., (2002) showed that professional musicians displayed larger Heschl's gyri than non-musicians.
On the other hand, for the pars opercularis, it was seen that every one of the individuals within the RSG had a right greater than left difference of grey-matter concentration. Thus, the anatomical asymmetries in this region seem to be more closely related to the functional asymmetry. This relationship may reflect a use-dependent reorganization. The left pars opercularis is related to speech production and is close to motor and premotor cortices. Many VBM studies have found volume increases in motor regions related to use-dependent factors. For example, Schlaug (2001) showed that the motor cortex region of musicians revealed grey-matter volume changes corresponding to the hand use of these musicians. Also, Penhune et al. (2003) found that deaf subjects, who use the right hand for signing, showed an increase of grey-matter density in their left motor hand region. Thus, we speculate that the morphological asymmetries observed here in the LSG, and its right-hemisphere homologue in the RSG, may also be use-dependent. In other words, patients with right-hemisphere speech may employ more the right motor-related regions in the expression of speech functions, and, conversely, patients with left-hemisphere speech may employ more the left motor-related regions.
Thus, in conclusion, whereas HG morphological asymmetry may be predetermined and more resistant to change, the pars opercularis morphological asymmetry may be related to use-dependent factors. Asymmetries in these two regions appear to bear a stronger relationship to language lateralization than the PT.
Supplementary material
Supplementary data are available at Brain Online.
We wish to thank Dr Alan Evans for making available the brain-imaging center facilities. We also thank Dr Marilyn Jones-Gotman for providing information on the Sodium Amytal data, and the staff of the Neuropsychology Unit for carrying out the IAP testing. This research work was supported by grants from the CIHR (Canadian Institutes of Health Research) and the McDonnell-Pew Cognitive Neuroscience program awarded to R.J.Z. as well as a FCAR (Formation de Chercheurs et l'Aide à la Recherche) scholarship to the first author.
References
Amunts K, Schleicher A, Bürgel U, Harmut M, Uylings HBM, Zilles K. Broca's region revisited: cytoarchitecture and intersubject variability.
Anderson B, Southern BD, Powers RE. Anatomic asymmetries of the posterior superior temporal lobes: a postmortem study.
Binder J, Frost J, Hammeke T, Rao S, Cox R. Function of the left planum temporale in auditory and linguistic processing.
Branch C, Milner B, Rasmussen T. Intracarotid sodium Amytal for the lateralization of cerebral speech dominance: observations in 123 patients.
Cappa SF, Perani D, Grassi F, Bressi S, Alberoni M, Franceschi M, et al. A PET follow up study of recovery after stroke in acute aphasics.
Cappa SF, Vallar G. The role of the left and right hemispheres in recovery from aphasia.
Chi JG, Dooling EC, Gilles FH. Left-right asymmetries of the temporal speech areas of the human fetus.
Collins D, Neelin P, Peters T, Evans A. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space.
Di Chiro G. Angiographic patterns of cerebral convexity veins and superficial dural sinuses.
Dos Santos Sequeira S, Woerner W, Walter C, Kreuder F, Lueken U, Westerhausen R, et al. Handedness, dichotic-listening ear advantage, and gender effects on planum temporale asymmetry—a volumetric investigation using structural magnetic resonance imaging.
Eckert MA, Leonard CM, Possing ET, Binder JR. Uncoupled leftward asymmetries for planum morphology and functional language processing. Brain and Language
Emmorey K, Allen JS, Bruss J, Schenker N, Damasio H. A morphometric analysis of auditory brain regions in congenitally deaf adults.
Foundas A, Leonard C, Gilmore R, Fennell E, Heilman K. Planum temporale asymmetry and language dominance.
Foundas AL, Eure KF, Luevano LF, Weinberger DR. MRI asymmetries of Broca's area: the pars triangularis and pars opercularis.
Geschwind N, Levitsky W. Human brain: left-right asymmetries in the temporal speech region.
Good CD, Johnsrude I, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains.
Griffiths T, Warren J. The planum temporale as a computational hub.
Honeycutt NA, Musick A, Barta PE, Pearlson GD. Measurement of the planum temporale (PT) on magnetic resonance imaging scans: temporal PT alone and with parietal extension.
Hutsler J, Gazzaniga M. Acetylcholinesterase staining in human auditory and language cortices—regional variation of structural features.
Jäncke L, Steinmetz H. Auditory lateralization and planum temporale asymmetry.
Jones-Gotman M. Intracarotid amobarbital testing in presurgical evaluation of patients with epilepsy.
Josse G, Mazoyer B, Crivello F, Tzourio-Mazoyer N. Left planum temporale: an anatomical marker on left hemispheric specialization for language comprehension.
Kulynych JJ, Vladar K, Jones DW, Weinberger DR. Gender differences in the normal lateralization of the supratemporal cortex: MRI surface-rendering morphometry of Heschl's gyrus and the planum temporale.
Liégeois F, Connelly A, Cross JH, Boyd SG, Gadian DG, Vargha-Khadem F, et al. Language reorganization in children with early-onset lesions of the left hemisphere: an fMRI study.
Loftus W, Tramo M, Thomas C, Green R, Nordgren R, Gazzaniga M. Three-dimensional quantitative analysis of hemispheric asymmetry in the human superior temporal region.
MacDonald J, Avis D, Evans A. Multiple surface identification and matching in magnetic resonance images.
Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, et al. Structural maturation of neural pathways in children and adolescents: in vivo study.
Penhune VB, Cismaru R, Dorsaint-Pierre R, Petitto L-A, Zatorre RJ. The morphometry of auditory cortex in the congenitally deaf measured using MRI.
Penhune VB, Zatorre RJ, MacDonald JD, Evans AC. Interhemispheric anatomical differences in human primary auditory cortex: probabilistic mapping and volume measurement from magnetic resonance scans.
Perria L, Rosadini G, Rossi G. Determination of side of cerebral dominance with amobarbital.
Pujol J, López-Sala A, Deus J, Cardoner N, Sebastián-Gallés N, Conesa G, et al. The lateral asymmetry of the human brain studied by volumetric magnetic resonance imaging.
Rademacher J, Caviness VS, Steinmetz H, Galaburda AM. Topographical variation of the human primary cortices: implications for neuroimaging, brain mapping and neurobiology.
Rademacher J, Morosan P, Schormann T, Schleicher A, Werner C, Freund HJ, et al. Probabilistic mapping and volume measurement of human primary auditory cortex.
Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions.
Ratcliff G, Dila C, Taylor L, Milner B. The morphological asymmetry of the hemispheres and cerebral dominance for speech: a possible relationship.
Risse GL, Gates JR, Fangman MC. A reconsideration of bilateral language representation based on the intracarotid amobarbital procedure.
Rubens AB, Mahowald MW, Hutton JT. Asymmetry of the lateral (sylvian) fissures in man.
Schlaug G. The brain of musicians. A model for functional and structural adaptation.
Schneider P, Scherg M, Dosch HG, Specht HJ, Gutschalk A, Rupp A. Morphology of Heschl's gyrus reflects enhanced activation in the auditory cortex of musicians.
Seldon HL. Structure of human auditory cortex I: cytoarchitectonics and dendritic distributions.
Seldon HL. Structure of human auditory cortex II: axon distributions and morphological correlates of speech perception.
Seldon HL. Structure of human auditory cortex III: statistical analysis of dendritic trees.
Shapleske J, Rossell SL, Woodruff PWR, David AS. The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance.
Steinmetz H, Rademacher J, Huang Y, Hefter H, Zilles K, Thron A, et al. Cerebral asymmetry: MR planimetry of the human planum temporale.
Steinmetz H, Volkmann J, Jäncke L, Freund H-J. Anatomical left-right asymmetry of language-related temporal cortex is different in left- and right-handers.
Strauss E, LaPointe JS, Wada JA, Gaddes W, Kosaka B. Language dominance: correlation of radiological and functional data.
Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers, Inc.;
Tallal P, Miller S, Fitch R. Neurobiological basis of speech: a case for the pre-eminence of temporal processing.
Thulborn KR, Carpenter PA, Just MA. Plasticity of language-related brain function during recovery from stroke.
Tisserand DJ, van Boxtel MP, Pruessner JC, Hofman P, Evans AC, Jolles J. A voxel- based morphometric study to determine individual differences in gray matter density associated with age and cognitive change over time.
Tomaiuolo F, MacDonald JD, Caramanos Z, Posner G, Chiavaras M, Evans AC, et al. Morphology, morphometry and probability mapping of the pars opercularis of the inferior frontal gyrus: an in vivo MRI analysis.
Tzourio N, Nkanga-Ngila B, Mazoyer B. Left planum temporale surface correlates with functional dominance during story listening.
von Economo C, Horn L. Über Windungsrelief, masse und Rindenarchitektonik der Supratemporalfläche, ihre individuellen und ihre Seitenunterschiede.
Wada JA, Clarke R, Hamm A. Cerebral hemispheric asymmetry in humans: cortical speech zones in 100 adult and 100 infant brains.
Watkins KE, Paus T, Lerch JP, Zijdenbos A, Collins DL, Neelin P, et al. Structural asymmetries in the human brain: a voxel-based statistical analysis of 142 MRI scans.
Westbury CF, Zatorre RJ, Evans AC. Quantifying variability in the planum temporale: a probability map.
Witelson S, Pallie W. Left hemisphere specialization for language in the newborn: neuroanatomical evidence of asymmetry.
Witelson SF, Glezer II, Kigar DL. Women have a greater density of neurons in the posterior temporal cortex.
Witelson SF, Kigar DL. Asymmetry in brain function follows asymmetry in anatomical form: gross, microscopic, postmortem and imaging studies. In: Boller F, Grafman J, editors. Handbook of neuropsychology. Vol 1. Amsterdam: Elsevier Science BV;
Worsley K, Marrett S, Neelin P, Vandal A, Friston K, Evans A. A unified statistical approach for determining significant signals in images of cerebral activation.
Zatorre RJ. Perceptual asymmetry on the dichotic fused words test and cerebral speech lateralization determined by the carotid sodium Amytal test.
Zatorre RJ, Belin P, Penhune VB. Structure and function of auditory cortex: music and speech.
Author notes
1Neuropsychology and Cognitive Neuroscience Unit and 2Brain Imaging Center, Montreal Neurological Institute, McGill University, 3Department of Psychology, Concordia University, Montreal, 4Mouse Imaging Center, Hospital for Sick Children, Toronto, Canada and 5Department of Experimental Psychology and FMRIB Center, University of Oxford, Oxford, UK