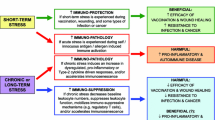
Abstract
The mechanisms that regulate neuronal function are a sum of genetically determined programs and experience. The effect of experience on neuronal function is particularly important during development, because early-life positive and adverse experience (stress) may influence the still “plastic” nervous system long-term. Specifically, for hippocampal-mediated learning and memory processes, acute stress may enhance synaptic efficacy and overall learning ability, and conversely, chronic or severe stress has been shown to be detrimental. The mechanisms that enable stress to act as this “double-edged sword” are unclear. Here, we discuss the molecular mediators of the stress response in the hippocampus with an emphasis on novel findings regarding the role of the neuropeptide known as corticotropin-releasing hormone (CRH). We highlight the physiological and pathological roles of this peptide in the developing hippocampus, and their relevance to the long-term effects of early-life experience on cognitive function during adulthood.
Similar content being viewed by others
References
Aamodt S. M. and Constantine-Paton M. (1999) The role of neural activity in synaptic development and its implications for adult brain function. Adv. Neurol. 79, 133–144.
Rubenstein J. L. R. and Rakic P. (1999) Genetic control of cortical development. Cereb. Cortex 9, 521–523.
Monuki E. S. and Walsh C. A. (2001) Mechanisms of cerebral cortical patterning in mice and humans. Nat. Neurosci. Suppl. 4, 1199–1206.
Roozendaal B., Quirarte G. L., and McGaugh J. L. (1997) Stress-activated hormonal systems and the regulation of memory storage. Ann. NY Acad. Sci. 821, 247–258.
Schafe G. E., Nader K., and Blair H. T. (2001) Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci. 24, 540–546.
Altman J. and Bayer S. A. (1990) Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J. Comp. Neurol. 301, 365–381.
Cameron H. A. and Gould E. (1996) The control of neuronal birth and survival, in Receptor Dynamics in Neural Development (Shaw C. A., ed.), CRC Press, New York, NY, pp. 141–157.
Bender R. A., Lauterborn J. C., Gall C. M., Cariaga W., and Baram T. Z. (2001) Enhanced CREB phosphorylation in immature dentate gyrus granule cells precedes neurotrophin expression and indicates a specific role of CREB in granule cell differentiation. Eur. J. Neurosci. 13, 679–686.
Amaral D. G. and Dent J. A. (1981) Development of mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansion. J. Comp. Neurol. 195, 51–86.
Ribak C. E. and Navetta M. S. (1994) An immature mossy fiber innervation of hilar neurons may explain their resistance to kainate-induced cell death in 15-day-old rats. Dev. Brain Res. 79, 47–62.
Tamamaki N. (1999) Development of afferent fiber lamination in the infrapyramidal blade of the rat dentate gyrus. J. Comp. Neurol. 411, 257–266.
Swann J. W., Smith K. L., and Lee C. L. (2001) Neuronal activity and the establishment of normal and epileptic circuits during brain development. Int. Rev. Neurobiol. 45, 89–118.
Liu D., Diorio J., Tannenbaum B., Caldji C., Francis D., Freedman A., et al. (1997) Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 277, 1659–1662.
Sanchez M. M., Hearn E. F., Do D., Rilling J. K., and Herndon J. G. (1998) Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res. 812, 38–49.
Kempermann G., Kuhn H. G., and Gage F. H. (1997) More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493–495.
Williams B. M., Luo Y., Ward C., Redd K., Gibson R., Kuczaj S. A., et al. (2001) Environmental enrichment: effects on spatial memory and hippocampal CREB immunoreactivity. Physiol. Behav. 73, 649–658.
Trickett P. K. and McBride-Chang C. (1995) The developmental impact of different forms of child abuse and neglect. Dev. Rev. 15, 311–337.
Bremner J. D., Randall P., Vermetten E., Staib L., Bronen R. A., Mazure C., et al. (1997) Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—a preliminary report. Biol. Psychiatry 41, 23–32.
Stein M. B., Koverola C., Hanna C., Torchia M. G., and McClarty B. (1997) Hippocampal volume in women victimized by childhood sexual abuse. Psychol. Med. 27, 951–959.
Sanchez M. M., Ladd C. O., and Plotsky P. M. (2001) Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Dev. and Psychopathol. 13, 419–449.
Flagel S. B., Vazquez D. M., Watson S. J. Jr., and Neal C. R. Jr. (2002) Effects of tapering neonatal dexamethasone on rat growth, neurodevelopment, and stress response. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282, 55–63.
Uno H., Eisele S., Sakai A., Shelton S., Baker E., DeJesus O., et al. (1994) Neurotoxicity of glucocorticoids in the primate brain. Horm. Behav. 28, 336–348.
Brunson K. L., Eghbal-Ahmadi M., Bender R., Chen Y., and Baram T. Z. (2001) Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc. Natl. Acad. Sci. USA 98, 8856–8861.
Meaney M. J., Aitken D. H., Van Berkel C., Bhatnagar S., and Sapolsky R. M. (1988) Effect of neonatal handling on age-related impairments associated with the hippocampus. Science 239, 766–768.
Keller-Wood M. and Dallman M. (1984) Corticosteroid inhibition of ACTH secretion. Endocr. Rev. 5, 1–24.
Sawchenko P. E. (1987) Evidence for a local site of action for glucocorticoids in inhibiting CRF and vasopressin expression in parvocellular neurosecretory neurons. Brain Res. 403, 213–224.
de Kloet E. R., De Kock S., Schild V., and Veldhuis H. D. (1988) Antiglucocorticoid RU 38486 attenuates retention of a behavior and disinhibits the hypothalamic-pituitary-adrenal axis at different sites. Neuroendocrinol. 47, 109–115.
Baram T. Z., Chalmers D. T., Chen C., Koutsoukos Y., and De Souza E. B. (1997) The CRF1 receptor mediates the excitatory actions of corticotropin releasing factor (CRF) in the developing rat brain: in vivo evidence using a novel, selective, non-peptide CRF receptor antagonist. Brain Res. 770, 89–95.
Herman J. P. and Cullinan W. E. (1997) Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 20, 78–84.
Lopez J. F., Akil H., and Watson S. J. (1999) Neural circuits mediating stress. Biol. Psychiatry 46, 1461–1471.
Baram T. Z. and Hatalski C. G. (1998) Neuropeptide-mediated excitability: a key triggering mechanism for seizure generation in the developing brain. Trends Neurosci. 21, 471–476.
Chen Y., Hatalski C. G., Brunson K. L., Baram T. Z. (2002) Rapid phosphorylation of the CRE binding protein precedes stress-induced activation of the corticotropin releasing hormone gene in medial parvocellular hypothalamic neurons of the immature rat. Mol. Brain Res. 96, 39–49.
Imaki T., Shibasaki T., Hotta M., and Demura H. (1993) Intracerebroventricular administration of corticotropin-releasing factor induces c-fos mRNA expression in brain regions related to stress responses: comparison with pattern of c-fos mRNA induction after stress. Brain Res. 616, 114–125.
McGaugh J. L., Cahill L., and Roozendaal B. (1996) Involvement of the amygdala in memory storage: interaction with other brain systems. Proc. Natl. Acad. Sci. USA 93, 13,508–13,514.
Hatalski C. G., Guirguis C., and Baram T. Z. (1998) Corticotropin releasing factor mRNA expression in the hypothalamic paraventricular nucleus and the central nucleus of the amygdala is modulated by repeated stress in the immature rat. J. Neuroendocrinol. 10, 663–669.
Hatalski C. G., Brunson K. L., Tantayanubutr B., Chen Y., and Baram T. Z. (2000) Neuronal activity and stress differentially regulate hippocampal and hypothalamic corticotropin releasing hormone expression in the immature rat. Neuroscience 101, 571–580.
Tannahill L. A., Sheward W. J., Robinson I. C., and Fink G. (1991). Corticotropin-releasing factor-41, vasopressin and oxytocin release into hypophysial portal blood in the rat: effects of electrical stimulation of the hypothalamus, amygdala and hippocampus. J. Endocrinol. 129, 99–107.
Beaulieu S., Pelletier G., Vaudry H., and Barden N. (1989) Influence of the central nucleus of the amygdala on the content of corticotropin-releasing factor in the median eminence. Neuroendocrinology. 49, 255–261.
Cullinan W. E., Helmreich D. L., and Watson S. J. (1996) Fos expression in forebrain afferents to the hypothalamic paraventricular nucleus following swim stress. J. Comp. Neurol. 368, 88–99.
Roozendaal B. and McGaugh J. L. (1997) Basolateral amygdala lesions block the memory enhancing effect of glucocorticoid administration in the dorsal hippocampus of rats. Eur. J. Neurosci. 9, 76–83.
Garcia R., Tocco G., Baudry M., and Thompson R. F. (1998) Exposure to a conditioned aversive environment interferes with long-term potentiation induction in the fimbria-CA3 pathway. Neuroscience 82, 139–145.
Blank T., Nijholt I., Eckart K., and Spiess J. (2002) Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: implications for hippocampus-dependent learning. J. Neurosci. 22, 3788–3794.
De Souza E. B., Insel T. R., Perrin M. H., Rivier J., Vale W. W., and Kuhar M. J. (1985) Corticotropin-releasing factor receptors are widely distributed within the rat CNS: an autoradiographic study. J. Neurosci. 5, 3189–3203.
Gray T. S. and Bingaman E. W. (1996) The amygdala: corticotropin-releasing factor, steroids, and stress. Crit. Rev. Neurobiol. 10, 155–168.
Eghbal-Ahmadi M., Hatalski C. G., Lovenberg T. W., Avishai-Eliner S. A., Chalmers D. T., and Baram T. Z. (1998) The developmental profile of the corticotropin releasing hormone receptor (CRF2) in rat brain predicts distinct agespecific functions. Dev. Brain Res. 107, 81–90.
Chen Y., Brunson K., Cariaga W., and Baram T. Z. (2000) Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 analysis using an antibody directed against the C-terminus. J. Comp. Neurol. 420, 305–323.
Avishai-Eliner S. A., Yi S. J., and Baram T. Z. (1996) Developmental profile of messenger RNA for the corticotropin-releasing hormone receptor in the limbic system. Dev. Brain Res. 91, 159–163.
Kalin N. H., Takahashi L. K., and Chen F-L (1994) Restraint stress increases corticotropin-releasing hormone mRNA content in the amygdala and paraventricular nucleus. Brain Res. 656, 182–186.
Merali Z., McIntosh J., Kent P., Michaud D., and Anisman H. (1998) Aversive and appetitive events evoke the release of corticotropin-releasing hormone and bombesin-like peptides at the central nucleus of the amygdala. J. Neurosci. 18, 4758–4766.
Swiergel A. H., Takahashi L. K., and Kalin N. H. (1993) Attenuation of stress-induced behavior by antagonism of corticotropin-releasing factor receptors in the central amygdala in the rat. Brain Res. 623, 229–234.
Roozendaal B., Brunson K. L., Holloway B. L., McGaugh J. L., Baram T. Z. (2002) Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc. Nat. Acad. Sci. 99, 13,908–13,913.
Piekut D. T. and Phipps B. (1998) Increased corticotropin-releasing factor immunoreactivity in select brain sites following kainate elicited seizures. Brain Res. 781, 99–111.
Swanson L. W., Sawchenko P. E., Rivier J., and Vale W. W. (1983) Organization of ovine corticotropin releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36, 165–186.
Sakanaka M., Shibasaki T., and Lederis K. (1987) Corticotropin releasing factor-like immunoreactivity in the rat brain as revealed by a modified cobalt-glucose oxidase-diaminobenzadine method. J. Comp. Neurol. 260, 256–298.
Yan X. X., Toth Z., Schultz L., Ribak C. E., and Baram T. Z. (1998) Corticotropin releasing hormone (CRH)-containing neurons in the hippocampal formation: morphological and neurochemical characterization. Hippocampus 8, 231–243.
Chen Y., Bender R. A., Frotscher M., and Baram T. Z. (2001) Novel and transient populations of corticotropin-releasing hormone-expressing neurons in developing hippocampus suggest unique functional roles: a quantitative spatiotemporal analysis. J. Neurosci. 21, 7171–7181.
Chugani H. T., Behen M. E., Muzik O., Juhasz C., Nagy F., and Chugani D. C. (2001) Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. Neuroimage 14, 1290–1301.
Vazquez D. M., Lopez J. F., Van Hoers H., Watson S. J., and Levine S. (2000) Maternal deprivation regulates serotonin 1A and 2A receptors in the infant rat. Brain Res. 855, 76–82.
Plotsky P. M. and Meaney M. J. (1993) Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol. Brain Res. 18, 195–200.
Avishai-Eliner S., Eghbal-Ahmadi M., Tabachnik E., Brunson K. L., and Baram T. Z. (2001) Down-regulation of hypothalamic corticotropin-releasing hormone messenger ribonucleic acid (mRNA) precedes early-life experience-induced changes in hippocampal glucocorticoid receptor mRNA. Endocrinology 142, 89–97.
Levine S. and Lewis G. (1959) Critical period for the effects of infantile experience on the maturation of a stress response. Science 129, 42–43.
Hess J. L., Denenberg V. H., Zarrow M., and Peiffer W. D. (1969) Modification of the corticosterone response curve as a function of handling in infancy. Physiol. Behav. 4, 102–109.
Meaney M. J., Diorio J., Francis D., Widdowson J., LaPlante P., Caldji C., et al. (1996) Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev. Neurosci. 18, 49–72.
Francis D. D. and Meaney M. J. (1999) Maternal care and the development of stress responses. Curr. Opin. Neurobiol. 9, 128–134.
Eghbal-Ahmadi M., Avisai-Eliner S., Hatalski C. G., and Baram T. Z. (1999) Regulation of the expression of corticotropin releasing factor receptor type 2 (CRF2) in the hypothalamus and amygdala of the immature rat. J. Neurosci. 19, 3982–3991.
Lopez J. F., Liberzon I., Vazquez D. M., Young E. A., and Watson S. J. (1999) Serotonin 1A receptor messenger RNA regulation in the hippocampus after acute stress. Biol. Psychiatry 45, 934–937.
Czeh B., Michaelis T., Watanabe T., Frahm J., de Biurrun G., van Kampen M., et al. (2001) Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc. Natl. Acad. Sci. USA 98, 12,796–12,801.
Viau V., Sharma S., Plotsky P. M., and Meaney M. J. (1993) The hypothalamic-pituitary-adrenal response to stress in handled and nonhandled rats: differences in stress-induced plasma ACTH secretion are not dependent upon increased corticosterone levels. J. Neurosci. 13, 1097–1105.
Meaney M. J. and Aitken D. H. (1985) The effects of early postnatal handling on hippocampal glucocorticoid receptor concentrations: temporal parameters. Brain Res. 354, 301–304.
Herman J. P., Patel P. D., Akil H., and Watson S. J. (1997) Localization and regulation of glucocorticoid and mineralcorticoid receptor messenger RNAs in the hippocampal formation of the rat. Mol. Endocrinol. 3, 3072–3082.
Joels M. and de Kloet E. R. (1992) Control of neuronal excitability by corticosteroid hormones. Trends Neurosci. 15, 25–30.
Pavlides C., Watanabe Y., Magarinos A. M., and McEwen B. S. (1995) Opposing roles of type I and type II adrenal steroid receptors in hippocampal long-term potentiation. Neuroscience 68, 387–394.
Joels M. (2001) Corticosteroid actions in the hippocampus. J. Neuroendocrinol. 13, 657–669.
Hollrigel G. S., Chen K., Baram T. Z., and Soltesz I. (1998) The pro-convulsant actions of corticotropin-releasing hormone in the hippocampus of infant rats. Neuroscience 84, 71–79.
Aldenhoff J. B., Gruol D. L., Rivier J., Vale W., and Siggins G. R. (1983) Corticotropin releasing factor decreases postburst hyperpolarizations and excites hippocampal neurons. Science 221, 875–877.
Lee E. H., Hung H. C., Lu K. T., Chen W. H., and Chen H. Y. (1992) Protein synthesis in the hippocampus associated with memory facilitation by corticotropin-releasing factor in rats. Peptides 13, 927–937.
Behan D. P., Heinrichs S. C., Troncoso J. C., Liu X. J., Kawas C. H., Ling N., et al. (1995) Displacement of CRF from its binding protein as a possible treatment for Alzheimer’s disease. Nature 378, 284–287.
Lee E. H., Huang A. M., Tsuei K. S., and Lee W. Y. (1996) Enhanced hippocampal corticotropin-releasing factor gene expression associated with memory consolidation and memory storage in rats. Chin. J. Physiol. 39, 197–203.
Chalmers D. T., Lovenberg T. W., and De Souza E. B. (1995) Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J. Neurosci. 15, 6340–6350.
Hatalski C. G. and Baram T. Z. (1997) Stress-induced transcriptional regulation in the developing rat brain involves increased cyclic adenosine 3′, 5′-monophosphate-regulatory element binding activity. Mol. Endocrinol. 11, 2016–2024.
Avishai-Eliner S., Brunson K. L., Sandman C. A., and Baram T. Z. (2002) Stressed-out, or in (utero)? Trends Neurosci 25, 518–524.
Chen Y, Bender R. A. Mews K., Adelmann G., Frotscher M., and Baram T. Z. (2002) The hippocampal CRH synpase: mismatched pre- and postsynaptic elements. Soc. Neurosci. Abs. 867.9.
Smith B. N. and Dudek F. E. (1994) Agerelated epileptogenic effects of corticotropin-releasing hormone in the isolated CA1 region of rat hippocampal slices. J. Neurophysiol. 72, 2328–2333.
Baram T. Z., Hirsch E., Snead O. C., and Schultz L. (1992) Corticotropin-releasing hormone-induced seizures in infant rats originate in the amygdala. Ann. Neurol. 31, 488–494.
Ehlers C. L., Henriksen S. J., Wang M., Rivier J., Vale W., and Bloom F. E. (1983) Corticotropin releasing factor produces increases in brain excitability and convulsive seizures in rats. Brain Res. 278, 332–336.
Marrosu F., Fratta W., Carrangiu P., Giagheddu M., and Gessa G. L. (1988) Localized epileptiform activity induced by murine CRF in rats. Epilepsia 29, 369–373.
Baram T. Z. and Ribak C. E. (1995) Peptide-induced infant status epilepticus causes neuronal death and synaptic reorganization. Neuroreport 6, 277–280.
Sperber E. F., Haas K. Z., Stanton P. K., and Moshe S. L. (1991) Resistance of the immature hippocampus to seizure-induced synaptic reorganization. Dev. Brain Res. 60, 88–93.
Haas K. Z., Sperber E. F., Opanashuk L. A., Stanton P. K., and Moshe S. L. (2001) Resistance of immature hippocampus to morphologic and physiologic alterations following status epilepticus or kindling. Hippocampus 11, 615–625.
Pihoker C., Cain S. T., and Nemeroff C. B. (1992) Postnatal development of regional binding of corticotropin-releasing factor and adenylate cyclase activity in the rat brain. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 16, 581–586.
Brunson K. L., Schultz L., and Baram T. Z. (1998) The in vivo proconvulsant effects of corticotropin releasing hormone in the developing rat are independent of ionotropic glutamate receptor activation. Dev. Brain Res. 111, 119–128.
Lyons M. K., Anderson R. E., and Meyer F. B. (1991) Corticotropin releasing factor antagonist reduces ischemic hippocampal neuronal injury. Brain Res. 545, 339–342.
Strijbos P. J., Relton J. K., and Rothwell N. J. (1994) Corticotropin-releasing factor antagonist inhibits neuronal damage induced by focal cerebral ischemia or activation of NMDA receptors in the rat brain. Brain Res. 656, 405–408.
Maecker H., Desai A., Dash R., Rivier J., Vale W., and Sapolsky R. (1997) Astressin, a novel and potent CRF antagonist, is neuroprotective in the hippocampus when administered after a seizure. Brain Res. 744, 166–170.
Pederson W. A., Wan R., Zhang P., and Mattson M. P. (2002) Urocortin, but not urocortin II, protects cultured hippocampal neurons from oxidative and excitotoxic cell death via corticotropin-releasing hormone receptor type I. J. Neurosci. 22, 404–412.
Sapolsky R. M., Krey L. C., and McEwen B. S. (1985) Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J. Neurosci. 5, 1222–1227.
Reul J. M. and de Kloet E. R. (1985) Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 117, 2505–2511.
McEwen B. S. (1999) Stress and hippocampal plasticity. Annu. Rev. Neurosci. 22, 105–122.
Leverenz J. B., Wilkinson C. W., Wamble M., Corbin S., Grabber J. E., Raskind M. A., et al. (1999) Effect of chronic high-dose exogenous cortisol on hippocampal neuronal number in aged nonhuman primates. J. Neurosci. 19, 2356–2361.
Shetty A. K. and Turner D. A. (1997) Fetal hippocampal cells grafted to kainate-lesioned CA3 region of adult hippocampus suppress aberrant supragranular sprouting of host mossy fibers. Exp. Neurol. 143, 231–145.
Sapolsky R. M. (1996) Stress, glucocorticoids, and damage to the nervous system: the current state of confusion. Stress 1, 1–19.
Dubé C., Brunson K. L., Nehlig A., and Baram T. Z. (2000) Corticotropin releasing hormone activates specific neuronal circuits, as indicated by c-fos expression and glucose metabolism. J. Cereb. Blood Flow Metab. 20, 1414–1424.
Riviello P., de Rogalski Landrot I., and Holmes G. L. (2002) Lack of cell loss following recurrent neonatal seizures. Dev. Brain Res. 135, 101–104.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brunson, K.L., Chen, Y., Avishai-Eliner, S. et al. Stress and the developing hippocampus. Mol Neurobiol 27, 121–136 (2003). https://doi.org/10.1385/MN:27:2:121
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/MN:27:2:121