Summary
This multicentre randomised double-blind 3- to 5-year trial was designed to assess whether initial therapy with cabergoline alone or in combination with levodopa prevents or delays the occurrence of long term motor complications in patients with early Parkinson’s disease.
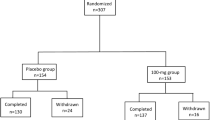
Patients eligible for study inclusion (n = 412) had early idiopathic Parkinson’s disease (Hoehn and Yahr stages 1 to 3) and had received no previous treatment with levodopa, selegiline or dopamine agonists. Patients were randomised to receive either cabergoline (0.25 to 4mg once daily) or levodopa (100 to 600 mg/day) titrated over a maximum period of 24 weeks. Once the optimum or maximum tolerated dose was achieved, it was maintained up to the end-point (development of motor complications confirmed at 2 consecutive 3-month visits) or up to a minimum of 3 years’ treatment. Open labelled levodopa was added in both treatment arms when the improvement in motor disability [Unified Parkinson’s Disease Rating Scale (UPDRS) factor III] decreased below 30% vs baseline. Both treatments improved motor disability, decreasing UPDRS factor III scores and factor II scores for activities of daily living.
The development of motor complications (end-point) was significantly less frequent in patients treated with cabergoline than in levodopa recipients (22% vs 34%; p < 0.02). The relative risk of developing motor complications during treatment with cabergoline was more than 50% lower than with levodopa.
Serious adverse events, either drug related or not, were slightly more frequent in cabergoline-treated patients (31%) than in those treated with levodopa (25%). The withdrawal rate in the cabergoline vs levodopa group was 16 vs 13%.
In conclusion, the study shows that, in patients with early Parkinson’s disease, cabergoline is effective either as monotherapy or combined with levodopa. Moreover, starting treatment with cabergoline significantly delays the development of motor complications.
Similar content being viewed by others
References
Fariello R, Carfagna N, Buonamici M, et al. Cabergoline: a long acting D2 agonist with antiparkinsonian properties: preclinical studies [abstract]. Ann Neurol 1991; 30: 258
Strolin-Benedetti M, Cocchiara G, Battaglia R, et al. Pharmacokinetic and metabolic pattern of cabergoline, a long-acting dopamine agonist in healthy volunteers. Presented at the 10th International Symposium on Parkinson’s Disease; 1991 Oct 19; Tokyo
Ferrari C, Barberi C, Caldara R, et al. Long-lasting prolactin-lowering effect of cabergoline, a new dopamine agonist in hyperprolactinemic patients. J Clin Endocrinol Metab 1986; 63: 941–5
Lera G, Vaamonde J, Muruzabal LM, et al. Cabergoline: a long-acting dopamine agonist in Parkinson’s disease. Ann Neurol 1990; 28: 593–4
Lees AJ. L-Dopa treatment and Parkinson’s disease. Q J Med 1986; 59: 535–47
Lesser RP, Fahn S, Snider SR, et al. Analysis of the clinical problems in parkinsonism and the complications of longterm levodopa therapy. Neurology 1979; 29: 1253–60
Rinne UK. Treatment of Parkinson’s disease: problems with a progressing disease. J Neural Transm 1981; 51: 161–74
Bravi D, Mouradian MM, Roberts JW, et al. Wearing-off fluctuations in Parkinson’s disease: contribution of postsynaptic mechanisms. Ann Neurol 36: 27–31, 1994
Leenders KL, Palmer AJ, Quinn N, et al. Brain dopamine metabolism in patients with Parkinson’s disease measured with positron emission tomography. J Neurol Neurosurg Psychiatry 1986; 49: 853–60
Rodriguez M, Lera G, Vaamonde J, et al. Motor response to apomorphine and levodopa in asymetric Parkinson’s disease. J Neurol Neurosurg Psychiatry 1994; 57: 562–6
Rinne UK. Early combination of bromocriptine and levodopa in the treatment of Parkinson’s disease: a 5-year follow-up. Neurology 1987; 37: 826–8
Stern G, Lees A. Long term effects of bromocriptine given to de novo patients with idiopathic Parkinson’s disease. In: Yahr MD, Bergmann KJ, editors. Adv Neurol Vol. 45. New York: Raven Press, 1986: 525–7
Hoehn M. Using bromocriptine to manage the inadequacies of L-Dopa therapy. In: Fahn S, Marsden CD, Jenner P, et al., editors. Recent developments in Parkinson’s disease. New York: Raven Press, 293–301, 1985
Rinne UK, Bracco F, Chouza C, et al. Cabergoline in the treatment of early Parkinson’s disease: results of the first year of treatment in a double-blind comparison of cabergoline and levodopa. Neurology 1997; 48: 363–88
Saint-Cyr JA, Taylor AE, Lang AE. Neuropsychological and psychiatric side effects in the treatment of Parkinson’s disease. Neurology 1993; 43 Suppl. 6: 547–52
van Dijk JG, Haan J, Zwinderman K, et al. Autonomic nervous system dysfunction in parkinson’s disease: relationships with age, medication, duration and severity. J Neurol Neurosurg Psychiatry 1993; 56: 1090–5
The Parkinson Study Group. The effect of deprenyl on the progression of disability in early Parkinson’s disease. N Engl J Med 1989; 321: 1364–71
Lees AJ, and the Parkinson’s Disease Research Group. Comparisons of therapeutic effects and mortality data of levodopa and levodopa combined with selegiline in patients with early mild Parkinson’s disease. BMJ 1995; 311: 1602–7
Rinne UK. Lisuride, a dopamine agonist in the treatment of early Parkinson’s disease. Neurology 1989; 39: 336–9
Montastruc JL, Rascol O, Senard JM, et al. A randomized controlled study comparing bromocriptine to which levodopa was later added, with levodopa alone in previously untreated patients with Parkinson’s disease: a five year follow-up. J Neurol Neurosurg Psychiatry 1994; 57: 1034–8
Giménez-Roldán S, Tolosa E, Burguera JA, et al. Early combination of bromocriptine and levodopa in Parkinson’s disease: a prospective randomized study of two parallel groups over a total follow-up period of 44 months including an initial 8-month double-blind stage. Clin Neuropharmacol 1997; 20: 67–76
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rinne, U.K., Bracco, F., Chouza, C. et al. Early Treatment of Parkinson’s Disease with Cabergoline Delays the Onset of Motor Complications. Drugs 55 (Suppl 1), 23–30 (1998). https://doi.org/10.2165/00003495-199855001-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199855001-00004