Abstract
Disruption of the dorsal frontostriatal pathways in Parkinson’s disease (PD) is associated with impairments in motivation, as well as in executive function. The goal of this study was to investigate whether these impairments are related and, if so, whether the disruption of frontostriatal pathways compromises the ability to process the motivational aspects of feedback in such tasks. In Experiment 1, informative feedback improved the performance of young, healthy participants in a task-switching paradigm. This task-switching paradigm was then used in Experiment 2 to test whether feedback would improve the performance of 17 PD patients and age-matched controls. The PD group benefitted from feedback to the same degree as control participants; however, depression scores on the Beck Depression Inventory were significantly related to feedback usage, especially when response selection demands were high. Regardless of feedback, PD patients were more impaired when response demands were high than in an equally difficult condition with low action demands. These results suggest that response selection is a core impairment of insufficient dopamine to the dorsal frontal striatal pathways.
Similar content being viewed by others
Cognitive impairments are consistently observed as a result of insufficient dopamine to frontostriatal pathways in Parkinson’s disease (PD). Although less visible than the motor and postural symptoms, cognitive impairments are not a trivial consequence of this disorder (Goldman, Baty, Buckles, Sahrmann, & Morris, 1998; Lees & Smith, 1983). Cognitive impairments are primarily in the realm of executive function and learning and are thought to arise because of deficient dopaminergic input from the basal ganglia to the prefrontal cortex (A. E. Taylor, Saint-Cyr, & Lang, 1986). As research into the cognitive sequelae of PD has progressed, another line of research has arisen that emphasizes an apparently different aspect of cognitive impairment. These studies have focused on motivational impairments in PD and, specifically, on abnormal responses to rewards (Czernecki, Pillon, Houeto, Pochon, Levy, & Dubois, 2002; Goerendt, Lawrence, & Brooks, 2004; Kunig, Leenders, Martin-Solch, Missimer, Magyar, & Schultz, 2000; Mazzoni, Hristova, & Krakauer, 2007). The goal of these experiments is to reconcile these seemingly disparate views of cognitive disorders in PD in order to understand the contribution of frontostriatal pathways to cognition.
Impairments of executive function have been well documented in PD over the last few decades. PD patients do poorly on executive function tests that require nonautomatic responding (A. E. Taylor et al., 1986), including tasks that require filtering the contents of working memory (Lee et al., 2010) and tests such as the Stroop task that require overcoming a prepotent response (Witt et al., 2006). In addition, those with PD are reliably impaired when required to switch quickly between multiple tasks (A. R. Cools, van den Bercken, Horstink, van Spaendonck, & Berger, 1984). Indeed, task switching has been a primary focus for investigations of executive function and PD. Task switching is a control process that produces a change in responding from one stimulus set, task, or rule to another (Monsell, 2003). Many studies have demonstrated that PD patients are slower at shifting than healthy controls. Greater shift costs were observed for patients, as compared with controls, when a switch was required between (1) responding to target stimuli in different modalities (Ravizza & Ivry, 2001), (2) responding to different stimulus features (i.e., shapes, letters) of a target (Hayes, Davidson, Keele, & Rafal, 1998; Pollux, 2004), (3) the type of information retrieved (i.e., make an odd/even judgment or a consonant/vowel judgment) (R. Cools, Barker, Sahakian, & Robbins, 2001a, Cools, Barker, Sahakian, & Robbins, 2001b; Rogers et al., 1998; Werheid, Koch, Reichert, & Brass, 2007), or (4) the rule for responding (Downes et al., 1989; Gauntlett-Gilbert, Roberts, & Brown, 1999; Joosten, Coenders, & Eling, 1995; Owen et al., 1993).
Further work has shown that it is the high response selection demands inherent in many tests of executive function that is problematic for those with PD. For example, task-switching deficits in PD are observed primarily when the switch requires a change in response sets or action plans (Helmich, Aarts, de Lange, Bloem, & Toni, 2009). A number of studies have now demonstrated that PD patients are impaired at task switching only when response selection demands are high (R. Cools, Barker, Sahakian, & Robbins, 2001a, Cools, Barker, Sahakian, & Robbins, 2001b; Ravizza & Ciranni, 2002), such as when the stimulus suggests two different response keys. Imaging studies have converged on these findings and have reported greater activity in the dorsal striatum in conditions where overcoming a prepotent response is necessary (Barber & Carter, 2004; Sylvester et al., 2003).
Executive functions such as task switching seem to be quite different in terms of their computational demands than is reward processing; however, the ability of rewards to motivate goal-directed behavior is also compromised in PD. Recent studies have reported that those with PD make less profitable decisions (Mimura, Oeda, & Kawamura., 2006) and are less sensitive to rewarding outcomes (Mazzoni et al., 2007; Rutledge et al., 2009). Neural activity in response to rewards is also atypical in those with PD (Goerendt et al., 2004; Kunig et al., 2000). These results converge with those of imaging studies showing that the basal ganglia regions affected in PD (the dorsolateral caudate and putamen; Kish, Shannak, & Hornykiewicz, 1988) are involved in reward processing. For example, several studies have shown that the activity in the dorsal striatum dissociates between rewards and punishments (Delgado, Locke, Stenger, & Fiez, 2003; Delgado, Nystrom, Fissell, Noll, & Fiez, 2000; Delgado, Stenger, & Fiez, 2004; Elliot, Newman, Longe, & William Deakin, 2004; Schonberg, Daw, Joel, & O’Doherty, 2007). These results are also consistent with studies of nonhuman primates that implicate dopamine neurons with reward prediction error (see Schultz, 2007, for a review).
In the present experiments, we investigated two hypotheses explaining concomitant executive function and reward processing deficits in PD. First, a primary deficit in reward processing may reduce motivation to perform well in a range of tasks, including executive function tasks. For example, one study of motor performance found that those with PD could perform just as quickly and accurately as healthy participants but chose to expend less energy because of a difference in their valuation of the costs and benefits of moving quickly (Mazzoni et al., 2007). One model of dopaminergic function suggests that dopamine neurons encode motivational salience that subsequently triggers cognitive control and action selection through projections to the striatum and dorsolateral prefrontal cortex (Bromberg-Martin, Matsumoto, & Hikosaka, 2010). It is possible, then, that differences in the value of rewards underlie executive function deficits in PD.
Note that most studies finding executive function impairments in PD did not link performance with monetary rewards. However, informative feedback about performance may also act as a reinforcer, and in fact, neural regions sensitive to monetary rewards are also responsive to informative feedback (Elliott et al., 2004; Tricomi, Delgado, McCandliss, McClelland, & Fiez, 2006). Thus, a difference in the sensitivity to the rewarding properties of informative feedback could underlie the executive function impairment observed in PD.
Executive functioning improves with monetary rewards (Gilbert & Fiez, 2004; Locke & Braver, 2008; S. F. Taylor et al., 2004; see Pessoa, 2009, for a review), but it is unknown whether purely informative feedback can improve performance. This gap in our knowledge is due to the long-held assumption that executive function is somewhat impervious to learning effects (Healy, Wohldmann, Sutton, & Bourne, 2006; although see Jaeggi, Buschkuehl, Jonides, & Perrig, 2008). In particular, no studies have directly examined whether the presentation of informative feedback affects task switching. If so, it is possible that a deficiency in processing the motivational properties of feedback accounts for observed differences between patients and controls in task switching. For example, a recent study of PD participants reported impaired feedback-based learning for those with co-morbid clinical depression (Herzallah et al., 2010). Depression is reported by 30%–40% of patients (Slaughter, Slaughter, Nichols, Holmes, & Martens, 2001) and may be linked to this diminished sensitivity to reward (Eshel & Roiser, 2010). Thus, depressive symptoms may modulate the way in which rewards are experienced by those with PD; in turn, this may produce a deficit in using the rewarding/punishing aspects of feedback as a motivator for improving performance.
An alternative hypothesis is that PD produces an impairment of response selection/inhibition that affects performance on executive function tasks and that is not modifiable by reward incentives. A shared finding in both the motivation and executive function literatures is the importance of response demands for cognitive impairment. In fMRI studies of reward processing, the dorsal striatum was sensitive to feedback only in the case where there was a choice between two possible responses (O'Doherty, Dayan, Schultz, Deichmann, Friston, & Dolan, 2004; Tricomi, Delgado, & Fiez, 2004). When only a single response was required, the dorsal striatum did not dissociate between positive and negative feedback. Similarly, many executive function tasks impose high demands on response selection/inhibition because of the need to overcome automatic responses to stimuli. For example, PD patients are impaired at attentional flanker tasks in which flankers and the targets suggest different responses (Wylie et al., 2009). In studies of task switching, shifting is slower only when two different responses are suggested by the target stimuli (R. Cools et al., 2001a, 2001b; Ravizza & Ciranni, 2002). An important shortcoming of these studies, however, is that the more difficult action condition has not been compared with an equally difficult nonaction condition. Without a proper control condition, it is difficult to determine whether PD patients are selectively impaired when response demands are high or whether the increase in difficulty produces a more sensitive measure of general deficits in task switching (Macdonald & Carter, 2002).
Overview of experiments
The hypothesis that informative feedback improves performance on executive function tasks has not been systematically tested. In order to assess whether problems in processing feedback underlie executive function impairments in PD, the first step is to determine whether feedback improves performance in participants without PD. In Experiment 1, we assessed the effect of feedback on young, healthy participants in a task-switching paradigm known to be difficult for those with PD (Hayes et al., 1998). We modified this paradigm in two ways: (1) We varied whether error-related feedback was presented after every trial, and (2) we added a condition in which shifting was difficult but response selection demands were low. In this way, we can test feedback effects in switching conditions that have high or low response demands and in which difficulty is matched.
In Experiment 2, we directly tested whether externally provided feedback can motivate performance for those with PD to the same extent as for age-matched controls while task switching. If problems with feedback processing underlie cognitive impairments in PD, feedback should have little impact in improving performance for those with PD. Moreover, we assessed whether executive function is impaired only when action demands are high by comparing performance in a control condition that was matched in switching difficulty but in which response demands were low.
Taken together, the results of these experiments will clarify whether informative feedback improves executive function and, if so, whether those with PD are impaired at these tasks because of problems with processing the motivational content of feedback. Moreover, our inclusion of a difficult condition with low response demands will allow us to determine whether task-switching deficits in PD are selectively related to impairments of response selection/inhibition.
Experiment 1
Feedback has not been systematically manipulated in previous studies of task switching in healthy participants. The effect of feedback on performance will be assessed for participants switching between shape and color identification. Of interest is whether performance will improve when feedback is provided.
Feedback was expected to be primarily motivational rather than instructive given that the experimental paradigm did not rely heavily on learning probabilistic stimulus–response contingencies. However, research showing that practice can improve task-switching speed in both younger and older adults may challenge this assumption (Karbach & Kray, 2009; Kray & Lindenberger, 2000). Moreover, those with PD are reliably impaired when learning requires feedback processing (Frank, Seeberger, & O’Reilly, 2004; Maddox, Aparicio, Marchant, & Ivry, 2005; Shohamy et al., 2004). Thus, we tested whether feedback was improving performance by increasing motivation or because feedback improved learning of the task.
Two manipulations of interference (response and stimulus) were included in this experiment to match switching performance under high response demands with an equally difficult condition that did not impose high demands on response selection and inhibition. In the latter case, switching difficulty was increased by making attentional selection more challenging. Previous studies have not had such a control for the response interference condition, which makes it difficult to know whether PD patients had problems with response competition per se or whether response competition just increased overall difficulty. An increase in overall difficulty might simply make subtle impairments in task switching more observable, without having anything to do with response selection/inhibition.
Method
Participants Thirty-eight undergraduates at Michigan State University participated in this experiment for course credit.
Stimuli The color set consisted of red and blue, and the set of shapes consisted of triangles and squares. The color red and the triangle were mapped to a left-key response, and the color blue and the square to a right-key response. On each trial, participants saw one of the colors inside one of the shapes (Fig. 1). Stimulus interference was increased by making both features equivalently bright and was reduced by making the task-irrelevant color or shape more transparent (Fig. 1b). Response interference was reduced by presenting stimuli that suggested the same response (e.g., red and triangle; congruent) or was enhanced by presenting stimuli that suggested different responses (e.g., blue and triangle; incongruent). Four types of competition (i.e., none, stimulus, response, both) were included within a block of trials (Fig. 1). In the stimulus interference condition, both features were salient (equivalently bright), and there was no response interference (Fig. 1b). In the response interference condition, incongruent stimuli were presented, but the relevant feature was brighter than the irrelevant feature (Fig. 1c). Performance in these two conditions was of primary interest ,whereas the none (Fig. 1a) and both (Fig. 1d) conditions were included to avoid habituation to the two critical conditions. In the both condition, both the shape and color were dim instead of bright, so that across the experiment, a dim stimulus was sometimes the target. Thus, dim stimuli could not be routinely ignored, thereby ensuring that response competition effects would be observed from the dim irrelevant feature.
Procedure Participants were required to switch between identifying colors or shapes that were presented in a compound figure (Fig. 1). The probability of a switch in the task set was .5. A cue was presented simultaneously with the compound figure and consisted of the word “COLOR” or “SHAPE” in Arial font (Fig. 2). The cue was always valid and instructed the participant as to the relevant feature. Once the color or shape of the relevant feature was identified, participants were asked to press the left key (red, triangle) or the right key (blue, square) to make a response. The left and right keys corresponded to the 0 and decimal keys on the number pad of the computer keyboard.
Informative and neutral feedback was presented for 500 ms after the response. In the informative feedback condition, the word “Correct” followed accurate trials, and the word “Incorrect” followed error trials. In the neutral feedback condition, the word “Ready” was presented after each trial. All feedback was presented in yellow Arial font. Feedback condition was blocked, and the order was counterbalanced across participants (ABBA or BAAB).
Participants practiced the color and shape mappings separately in blocks of 24 trials each, with feedback. If a 90% accuracy rate was not reached by the end of the practice block, practice continued for another 24 trials until the participant achieved this criterion. After practice, participants were tested in four blocks of 96 trials each in the task-switching experiment.
Results
Reaction time (RT) was analyzed using a 2 (stimulus/response interference) × 2 (neutral/informative feedback) × 2 (repeat/shift) repeated measures ANOVA. RTs on incorrect trials and those following incorrect trials were discarded. RTs longer than 5 s or greater/less than 3 standard deviations away from the participant’s average RT were excluded from the analysis (0%–7% of correct trials).
All main effects were significant (Fig. 3). Repeat trials were faster than shift trials, F(1, 37) = 28.3, p < .05, η 2p = .43), trials were faster in informative feedback blocks than with neutral feedback, F(1, 37) = 10.98, p < .05, η 2p = .23, and stimulus interference trials were faster than response interference trials, F(1, 37) = 17.22, p < .05, η 2p = .32. Interference did not interact with shifting [shift × interference, F(1, 37) = 2.13, p = .153, η 2p = .05; shift × interference × feedback, F(1, 37) = 0.17, p = .682, η 2p = .01. Despite the generally longer RTs in the response interference condition, shifting speed was equivalent in the two types of interference conditions. Thus, shift cost was equated between response and stimulus interference.
RT in Experiment 1 for young participants in the response and stimulus interference conditions when feedback was present or absent and when the trial switched or repeated from the previous trial
Feedback did not interact with type of interference, F(1, 37) = 0.53, p = .473, η 2p = .01. Thus, feedback was beneficial for improving speed in both stimulus and response interference conditions.
A significant interaction of feedback and shifting was observed, F(1, 37) = 5.89, p < .05, η 2p = .14. Paired-sample t-tests were used to assess the simple effects between cells. Participants were faster on shift trials when feedback was present, as compared with the absence of feedback, t(37) = 3.65, p < .05. In contrast, repeat trials were not reliably affected by feedback, t(37) = 1.78, p = .083.
Given the longer RTs in the response interference condition, we corrected for speed by transforming the average RT in each condition into a z-score (Faust, Balota, Speiler, & Ferraro, 1999). The analysis described above was run on the z-transformed data, and none of the results changed.
Accuracy rates were not compared between stimulus and response interference conditions. Accuracy is not a reliable marker of switching performance in the stimulus interference condition, because the response is identical for both sets. Thus, an error would not be detected if a participant failed to switch tasks. (Note, however, that a reliable shift cost in RT was observed in the stimulus interference condition, suggesting that participants were switching between task sets.) Only errors in the response interference condition were measured, and a significant error cost was observed, F(1, 37) = 17.83, p < .05, η 2p = .33. The interaction of feedback and shifting did not reach significance, F(1, 37) = 3.72, p = .061, η 2p = .09.
Discussion
Feedback was effective in reducing shift cost for young, healthy participants. Shift cost was lower when error informative feedback was provided than when such feedback was absent. Given this result, it is possible that the executive function impairments observed in PD are due to a deficit in processing feedback. This hypothesis was tested directly in Experiment 2.
Previous research has demonstrated that shift cost can be lowered with practice (Karbach & Kray, 2009; Kray & Lindenberger, 2000), suggesting that some form of learning occurs in the context of task switching. In the present experiment, error feedback was provided to participants; however, errors were not very frequent (4%). Thus, most feedback was positive, confirming that the participant had performed accurately. Given that there was little to learn over the course of the experiment, the benefit of feedback on performance is likely due to its motivational properties; that is, participants most likely experienced the informative feedback as a reinforcer that motivated them to do well.
Given that informative feedback may be acting as a positive reinforcer in task switching, it is possible PD patients do poorly in executive function paradigms because of a deficit in processing rewarding information. In a number of imaging studies, the dorsal striatum was shown to dissociate between rewards and punishments in gambling tasks where outcomes were random (Delgado et al., 2003; Delgado et al., 2000; Delgado et al., 2004). Moreover, the prevalence of depression symptoms is high in PD (30%–40%), suggesting that positive and negative reinforcers may not be processed effectively (Eshel & Roiser, 2010). The relationship of depression, feedback processing, and executive function will be examined in the next experiment.
Our second goal was to assess whether the stimulus interference condition was matched in difficulty to the response interference condition. We found that shift cost and feedback effects were equivalent in the two conditions. However, overall, RT was higher in the response competition condition, indicating that response interference was more difficult regardless of shifting. Importantly, this effect was not replicated with older adults.
Experiment 2
In Experiment 1, feedback reduced shift cost. This allows for the possibility that executive function deficits in PD are due to problems with processing the rewarding aspects of feedback. This experiment will assess these patients’ ability to switch tasks in the presence and absence of feedback. Moreover, participants rated their level of depression so that we could observe whether feedback-processing deficits were related to this factor. The inclusion of the stimulus interference condition allowed us to observe whether task-switching impairments were present solely when response selection demands werehigh or whether PD patients would show deficits in any difficult task-switching condition.
If the underlying deficit in executive function is due to a loss of sensitivity to the rewarding properties of informative feedback, PD patients should show less of an improvement in performance than controls when corrective feedback is provided. Alternatively, a core impairment in response selection and inhibition predicts that those with PD will be impaired in the response interference condition, regardless of feedback.
Method
Participants Seventeen patients with idiopathic PD and 17 healthy, matched controls participated in this experiment (see Table 1 for demographic data). They were paid for their participation at the rate of $10/h. All participants had normal or corrected vision and hearing, fluency in English, and no history of other neurological or psychiatric disorders. All PD patients (1) were in the mild-to-moderate stages of the disease, (2) were on dopaminergic therapy (see Table 1), (3) were stable on their doses for at least 3 months, and (4) showed no evidence of diffuse Lewy body disease. All patients were tested on their normal medication schedules. The majority of patients (n = 14) were taking levodopa/carbidopa. Three patients were taking only dopamine receptor agonists.
All participants scored above 23 on the Mini-Mental State Exam (MMSE) and in the minimal range (0–13) for depression on the Beck Depression Inventory II (BDI–II). All participants were given the Digit Span forward subtest of the Wechsler Adult Intelligence Test (IV) to assess each group’s ability to memorize stimulus–response mappings. It was confirmed with t-tests that patients and controls were matched on age, years of education, MMSE scores, and Digit Forward Span (all p-values > .5). Groups were not matched on their scores on the BDI–II, however. The PD group had significantly higher scores on this self-report measure of depression than did controls, t(32) = 3.72, p < .05. Note, however, that all individuals fell in the range of “minimal” depression, as assessed by this scale, and were not clinically depressed.
Procedure The procedure was similar to that in Experiment 1, except that participants were tested on a laptop computer.
Results
The same criteria as those used in Experiment 1 for excluding deviant RTs were implemented here. This resulted in the discarding of 0%–11% of RTs on correct trials. As an initial test to assess whether the stimulus and response competition conditions were matched, RTs were analyzed using a 2 (stimulus/response interference) × 2 (feedback) × 2 (shift) repeated measures ANOVA for just the older control group (Fig. 4a). Main effects of shifting, F(1, 16) = 32.03, p < .05, η 2p = .67, and interference, F(1, 16) = 20.96, p < .05, η 2p = .57, were observed. In contrast to younger adults, older participants were faster in the presence of response interference than in the presence of stimulus interference. The main effect of feedback was not significant, F(1, 16) = 0.84, p = .373, η 2p = .05, nor did feedback interact with other factors [feedback × interference, F(1, 16) = 0.01, p = .943, η 2p = .00; feedback × shift, F(1, 16) = 0.63, p = .44, η 2p = .04; feedback × interference × shift, F(1, 16) = 0.42, p = .53, η 2p = .03. The results stayed the same when RTs were transformed to z-scores.
Top: RT in Experiment 2 for older participants and PD patients in the response and stimulus interference conditions when feedback was present or absent and when the trial switched or repeated from the previous trial. Bottom: RT collapsed across feedback and switching in each group. RTs are raw means unadjusted for the effects of the covariate
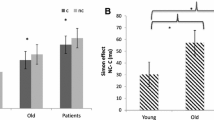
Given that depression is related to motivational deficits (Eshel & Roiser, 2010), BDI–II scores were used as a covariate of interest to observe whether these scores interacted with any of the independent variables. For the group comparison, a 2 (stimulus/response competition) × 2 (feedback) × 2 (shift) × 2 (group) repeated measures ANCOVA was performed on RTs, using BDI–II scores as a covariate (Fig. 4a). There was no main effect of group, F(1, 31) = 0.61, p = .439, η 2p = .02, indicating that both groups were generally matched on speed. However, the groups were differentially sensitive to the type of interference in the display, and a group × interference interaction was observed, F(1, 31) = 5.09, p < .05, η 2p = .14. Paired-sample t-tests indicated that healthy participants were significantly faster in the response interference condition than in the stimulus interference condition, t(16) = 4.54, p < .05, whereas PD patients did not reliably show this difference, t(16) = 1.19, p = .251 (Fig. 4b). No other group effects were significant. These results did not change in the analysis of the z-transformed data.
The ANCOVA indicated that depression scores on the BDI–II interacted with interference and feedback, F(1, 31) = 4.29, p < .05, η 2p = .12, although this effect became unreliable when z-transformed data were used, F(1, 31) = 3.08, p = .09, η 2p = .09. In order to understand this relationship with BDI scores, RT in the feedback condition was subtracted from RT in the no-feedback condition to create a difference score. This was done separately for the stimulus and response interference conditions. These difference scores (feedback effect) were then correlated with BDI–II scores (Fig. 5). Given that group did not significantly interact with this effect, all participants’ depression scores were analyzed. Feedback effects in the response competition condition were significantly related to depression, r(33) = -.38, p < .05; that is, less depressed participants were better able to use feedback to improve their speed of responding. In contrast, feedback was not significantly related to depression in the stimulus competition condition, r(33) = -.07, p = .667. To ensure that the significant correlation between BDI–II scores and feedback in the response competition condition was not driven by outliers, the Mahalanobis distance of this correlation was computed for each participant. The Mahalanobis distance was considered an outlier if it exceeded a critical value as determined by the chi-square distribution with 1 degree of freedom. No outliers were identified using this criterion.
Accuracy in the response competition condition was examined using BDI–II scores as a covariate, and a significant error cost was observed, F(1, 31) = 5.09, p < .05, η 2p = .14. Neither the effects of feedback and group nor the interaction of these factors with shifting was significant (p-values > .1).
Younger versus older adults The performance of older adults was dissociable from younger adults in two ways. First, older adults seemed unaffected by the presence of feedback even when their data were analyzed separately from those of the PD group. Second, older adults were faster in the response competition condition than in the stimulus condition, whereas younger adults showed the opposite pattern. To test this comparison more formally, we ran a repeated measures ANOVA with competition, feedback, and shifting as within-subjects factors to observe whether group interacted with any of these variables. This analysis was run with both raw and z-transformed means, with no change in the results. Consistent with other findings (Keys & White, 2000; Kramer, Hahn, & Gopher, 1999; Ravizza & Cirrani, 2002; Robbins et al., 1998), the older group had a significantly higher shift cost than did the younger group [shift × group, F(1, 53) = 13.52, p < .05, η 2p = .2]. However, the lack of a significant feedback × group interaction effect, F(1, 53) = 0.46, p = .5, η 2p = .01, indicates that the difference in feedback effects was not reliable when younger and older adults were compared, even though the older adults did not show a main effect of feedback when analyzed separately.
The difference in the effect of interference was confirmed by a significant interaction effect, F(1, 53) = 34.7, p < .05, η 2p = .4. Younger adults were 82 ms faster in the stimulus interference condition, whereas older adults were 120 ms faster in the response competition condition, t(53) = 5.86, p < .05. This difference was not due to a speed–accuracy trade-off in the response competition condition for older adults. For example, older adults might have been relatively faster in the response than in the stimulus interference condition because they emphasized speed in this condition, rather than accuracy. However, accuracy was generally higher for older adults (range of 97%–99% across conditions) than for younger adults (95%–99%).
Given the difference in stimulus and response interference effects in each group, we performed separate 2 (interference) × 2 (group) ANOVAs to assess the magnitude of these effects, as compared with the no-interference condition. The interaction of interference and group was significant for the comparison of response versus no interference, F(1, 53) = 6.0, p < .05, η 2p = .1, and stimulus versus no interference, F(1, 53) = 15.4, p < .05, η 2p = .23. The younger group was significantly slower in the response interference condition, t(37) = 5.1, p < .05, but not in the stimulus interference condition, t(37) = 0.42, p = .68, as compared with the no-interference condition. The older adults showed the opposite pattern; that is, they were slower in the stimulus interference condition, as compared with baseline, t(16) = 4.73, p < .05, but performance in the response interference condition was equivalent to that in the no-interference condition, t(53) = 1.06, p = .3.
Discussion
PD is associated with cognitive deficits in executive function and goal-oriented motivation. This experiment tested whether impairments of motivation or response selection were the cause of executive deficits in PD. We found that PD was not associated with impairments in processing informational feedback. Instead, PD was associated with problems in overcoming response interference regardless of feedback. While feedback processing was intact, as compared with age-matched controls, neither group displayed the feedback advantage observed in younger adults. However, this apparent aging effect may be complicated by the higher occurrence of depression in older adults. Level of depression was related to the beneficial effects of feedback in the response interference condition; that is, lower levels of self-rated depression were associated with greater effects of feedback. Taken together, these results suggest that executive function deficits linked to insufficient dopamine to dorsal frontostriatal pathways are not due to diminished motivation but may be impaired in those participants with higher levels of depression.
Consistent with previous research, resolving response interference was difficult for those with PD. Importantly, this result was not due to a greater level of difficulty in the response interference condition. Age-matched control participants were faster at resolving response interference than at resolving stimulus interference. Thus, PD was associated with a selective deficit in response selection that was not a consequence of general difficulty. These results suggest that problems with response selection and inhibition underlie both cognitive impairment in executive function and the motor consequences of PD.
Shift cost was unaffected by PD; instead, RT in both repeat and shift trials was affected by response interference. This result is surprising given that PD patients had a shift cost in this paradigm even while they were tested on medication in a previous study (Hayes et al., 1998). To more directly compare our results with those of this study, we assessed performance in the both condition, given that both stimulus and response interference were present in the Hayes et al. (1998) study; that is, all shapes and colors were bright. Error feedback was provided in that study as well. Shift cost tended to be higher for PD patients than for controls when both types of interference were present in instructive feedback blocks (174 vs. 126 ms), although this effect was far from reliable, t(32) = 0.46, p = .634. Our modified version of this paradigm was possibly more difficult than that used by Hayes et al. (1998), given that both our group of PD patients and control participants were almost twice as slow as participants in that study. One difference between the studies is that our stimuli changed more from trial to trial (e.g., both dim, both bright, bright shape/dim color). It is possible that the increased difficulty made response selection impairments more evident on both repeat and shift trials. This would have the effect of decreasing shift cost for PD patients while increasing the overall effect of response congruity. Note that the Hayes et al. (1998) study also reported that PD patients were especially impaired when responses were incongruent, although they did not report how this effect interacted with shifting.
This result is consistent, however, with fMRI studies showing greater involvement of the dorsal striatum in response competition than in task switching (Hedden & Gabrieli, 2010; Sylvester et al., 2003). For example, a recent study reported that the caudate and putamen were more engaged on repeat trials when response interference was present than on switch trials with no response interference (Hedden & Gabrieli, 2010). Moreover, deep brain stimulation of the subthalamic nucleus for those with PD improves their performance on a response inhibition (stop signal) task that does not include task switching (van den Wildenberg et al., 2006). Task switching and other executive functions often involve overcoming a prepotent response or choosing among competing response alternatives. Our results and others suggest that cognitive impairments will arise in PD when demands on the action system are high. Note, however, that these fMRI studies target only the dorsal striatum, whereas dysfunction is not selective to this region in PD.
This deficit in resolving response interference, but not stimulus interference, was unrelated to the presence or absence of feedback. Thus, problems with response selection and inhibition are not caused by deficits in processing the motivational aspects of feedback. However, it is possible that the frontostriatal network could be sensitive to the interaction of feedback and response selection. For example, an fMRI study of sequence learning tested whether monetary feedback modulated activity of the dorsal striatum that is often observed when the implicit learning of a repeating sequence of moves is contrasted with performing a series of random movements (Wachter, Lungu, Liu, Willingham, & Ashe, 2009). Activity of the putamen was observed in the absence of monetary or informative feedback; however, the putamen responded to an even greater degree for blocks in which rewarding feedback was provided.
The similar effects of feedback may be due to the fact that PD patients were tested on their normal medication schedule. Indeed, others have shown that medicated patients are better able to process positive reinforcers (Frank et al., 2004); thus, motivational impairments in executive function tasks may be more apparent in unmedicated patients. Note, however, that executive function deficits were observed here and in other studies when patients were tested on medication (Cools, Barker, Sahakian, & Robbins, 2001b; Hayes et al., 1998; Ravizza & Ivry, 2001). If an impairment of feedback processing were driving these executive function impairments, deficits of motivation should still be observed while the patients were on medication. Our results suggest that executive function impairments are more related to a compromised response selection system, rather than reduced sensitivity to feedback as a positive reinforcer.
Feedback effects were not observed in both groups of older adults; however, age-differences in feedback processing were unreliable when the younger and older control groups were compared. This may be due to power issues, given that the sample size of the older group was half that of the younger. Note, however, that the effect size of feedback in younger adults was on the order of .23 (partial eta squared); that is, feedback effects were not trivial, suggesting that they might have been observed in a smaller group. Alternatively, a potentially higher prevalence rate of depression in the older group may create a situation where sensitivity to reward is more variable in this group than in the younger group. A larger scale study in which depression symptoms were assessed in both groups is critical for addressing this question.
An unexpected result was that the younger group was slower when response interference was present, whereas the older group was slower in the stimulus interference condition. There is some support that older adults are more vulnerable to stimulus than to response interference. One study reported equivalent effects of response competition between younger and older adults, whereas older adults were more vulnerable to perceptual interference from task-relevant distractors (Wright & Elias, 1979). At small set sizes, older adults were particularly vulnerable to perceptual interference, but older and younger adults showed equivalent interference effects at larger set sizes (Maylor & Lavie, 1998). Given that our compound stimuli were composed of a single shape and color, the small set size may have induced an increased vulnerability to stimulus interference in the older group.
While our results are consistent with studies reporting greater stimulus interference effects in older adults, it is important to note a potential confound in the stimulus interference condition. Given that this was the only condition consisting of two bright stimuli, participants may have learned the contingency between brightness and the absence of response interference; that is, they may have learned that they did not need to use the cue when both stimuli were bright, because responding to either the color or the shape would result in a correct response. The presence of a reliable shift cost in this condition argues that participants were not using this strategy. The fact that participants were slower when the task set changed is not consistent with the idea that participants were responding randomly to either the color or the shape without using the cue. Nonetheless, this contingency may have been noticed by participants and may have affected their performance.
General discussion
Informative feedback was shown in Experiment 1 to improve executive function by acting as a positive reinforcer for young adults. However, executive impairments in PD were not selectively related to deficits in using feedback but were related to the level of response selection demand. While the PD group did not show an effect of feedback, this was true for older controls without PD as well. Deficits in using feedback to improve performance were linked to depressive symptoms in both the PD and older control groups.
The difference between the younger and older groups does not seem to be an effect of age per se. Using age as a covariate, rather than BDI–II scores, significant interactions of age and feedback were not observed (all p-values > .1). Instead, feedback effects were related to the level of reported depression, and depression levels were not significantly related to age, r(34) = .03, p = .858. Studies of the relationship of feedback processing for those with major depression or depressive symptoms have reported abnormal reactions to feedback, including enhanced reactivity to negative feedback (Santesso et al., 2008), a lack of posterror slowing (Steele, Kumar, & Ebmeier, 2007), hypoactivation of the anterior cingulate and the ventral striatum (Steele et al. 2007), and lower caudate volumes associated with anhedonic symptoms of depression (Pizzagalli et al., 2009). The latter results suggest a possible involvement of the basal ganglia in depression, as well as in response selection/inhibition (see Eshel & Roiser, 2010, for a review). While the PD group seems to have response selection impairments that are separable from depressive symptoms, it is possible that dopamine pathways less affected by PD may be related to depression and feedback processing. For example, dopamine loss is primarily observed in the dorsal frontostriatal pathways, rather than in the ventral striatal-orbitofrontal system (Kish et al., 1988). As PD progresses, these more ventral dopamine pathways may become disturbed. In fact, we found that patients who have had PD for a longer period of time had higher rated levels of depression. It is also possible that feedback impairments are due to disruption of serotonergic pathways. Associated with major depression, serotonin metabolism was altered in a subset of patients with PD (Mayeux et al., 1984), and research into the effects of serotonergic medication in PD is thought to hold promise for relieving mood disorders (Fox, Chuang, & Brotchie, 2009). Thus, motivation may involve the frontostriatal system but, perhaps, not the pathways that are primarily affected in PD.
Our results support the idea that impairments of executive function in PD are due to the high demands placed on response selection that are typical to such tasks. It is unclear, however, whether a compromised action system can explain deficits in other cognitive domains. For example, a reliable finding in the literature is that PD patients have problems using the information provided by feedback to guide their responses apart from their diminished sensitivity to the motivational properties of feedback. PD patients have difficulty learning classification and response rules through trial and error—a situation in which feedback is critical in linking actions with their consequences (Knowlton, Mangels, & Squire, 1996; Maddox et al., 2005; Shohamy et al., 2004; although see Osman, Wilkinson, Beigi, Castaneda, & Jahanshahi, 2008). Importantly, patients are able to learn classification and response rules when relying on observation (Shohamy et al., 2004). The feedback provided in the present study was purely motivational and did not affect learning across the experiment, so we cannot assess whether learning from feedback is separate from an impairment of response selection. However, we suggest that the dorsal striatum and prefrontal cortex may be heavily implicated in feedback-based learning, because this type of learning typically relies on action-based learning. In other words, participants not only have to process feedback, but also have to make a choice between two or more possible response alternatives. In contrast, response selection demands are minimal when observation, rather than feedback, is used; that is, learning proceeds by memorizing associated information while observing, rather than producing, a response. Thus, PD patients may be impaired at feedback-based learning because it involves response selection, whereas observational learning is intact in these patients because response selection demands are minimal in that condition. An important next step in assessing the pervasiveness of response selection deficits in PD is to observe whether feedback learning improves if action demands are lowered.
Executive function, motivational, and learning impairments are observed in PD patients when on medication (R. Cools et al., 2001a; Hayes et al, 1998; Mazzoni et al., 2007; Ravizza & Ivry, 2001; Shohamy et al., 2004), although such deficits are worse when patients are tested off their medication (R. Cools et al., 2001b; Czernecki et al., 2002; Hayes et al., 1998; Rutledge et al., 2009). It has been conjectured that dopaminergic medication restores functioning to the dorsal frontostriatal pathways, which improves motor symptoms and, perhaps, cognitive and motivational functions that rely on this dorsal network. While we still observed a response selection deficit in PD patients, both older controls and PD patients were not affected by the presence of feedback; that is, compromised motivational functioning due to age-related neuronal changes is equivalent to motivational impairments in medicated PD patients. If we had tested patients off their medication, a true difference in motivation between groups may have been unmasked and may have increased our sensitivity to probing motivational deficits. Although reward sensitivity did not differ between older controls and PD patients tested off their medication cycle in a previous study (Rutledge et al., 2009), these results should be replicated with a group of PD patients tested off their medication.
Our results suggest that the neural mechanisms by which motivational feedback improves executive function are likely to reside in neural regions less affected by PD. The impairment of executive function observed in PD is more likely to be due to problems in resolving response interference as a result of the loss of dopamine in pathways connecting the dorsal striatum and prefrontal/premotor cortices.
References
Barber, A. D., & Carter, C. S. (2004). Cognitive control involved in overcoming prepotent response tendencies and switching between tasks. Cerebral Cortex, 15, 899–912.
Bromberg-Martin, E. S., Matsumoto, M., & Hikosaka, O. (2010). Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron, 68, 815–834.
Cools, A. R., van den Bercken, J. H., Horstink, M. W., van Spaendonck, K. P., & Berger, H. J. (1984). Cognitive and motor shifting aptitude disorder in Parkinson's disease. Journal of Neurology, Neurosurgery, and Psychiatry, 47, 443–453.
Cools, R., Barker, R. A., Sahakian, B. J., & Robbins, T. W. (2001a). Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cerebral Cortex, 11, 1136–1143.
Cools, R., Barker, R. A., Sahakian, B. J., & Robbins, T. W. (2001b). Mechanisms of cognitive set flexibility in Parkinson's disease. Brain, 124, 2503–2512.
Czernecki, V., Pillon, B., Houeto, J. B., Pochon, J. B., Levy, R., & Dubois, B. (2002). Motivation, reward, and Parkinson's disease: Influence of dopatherapy. Neuropsychologia, 40, 2257–2267.
Delgado, M. R., Locke, H. M., Stenger, V. A., & Fiez, J. A. (2003). Dorsal striatum responses to reward and punishment: Effects of valence and magnitude manipulations. Cognitive, Affective, & Behavioral Neuroscience, 3, 27–38.
Delgado, M. R., Nystrom, L. E., Fissell, C., Noll, D. C., & Fiez, J. A. (2000). Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology, 84, 3072–3077.
Delgado, M. R., Stenger, V. A., & Fiez, J. A. (2004). Motivation-dependent responses in the human caudate nucleus. Cerebral Cortex, 14, 1022–1030.
Downes, J. J., Roberts, A. C., Sahakian, B. J., Evenden, J. L., Morris, R. G., & Robbins, T. W. (1989). Impaired extra-dimensional shift performance in medicated and unmedicated Parkinson's disease: Evidence for a specific attentional dysfunction. Neuropsychologia, 27, 1329–1343.
Elliott, R., Newman, J. L., Longe, O. A., & William Deakin, J. F. (2004). Instrumental responding for rewards is associated with enhanced neuronal response in subcortical reward systems. NeuroImage, 21, 984–990.
Eshel, N., & Roiser, J. P. (2010). Reward and punishment processing in depression. Biological Psychiatry, 68, 118–124.
Frank, M. J., Seeberger, L. C., & O'Reilly, R. C. (2004). By carrot or by stick: Cognitive reinforcement learning in Parkinsonism. Science, 306, 1940–1943.
Gauntlett-Gilbert, J., Roberts, R. C., & Brown, V. J. (1999). Mechanisms underlying attentional set-shifting in Parkinson's disease. Neuropsychologia, 37, 605–616.
Gilbert, A. M., & Fiez, J. A. (2004). Integrating rewards and cognition in the frontal cortex. Cognitive, Affective, & Behavioral Neuroscience, 4, 540–552.
Goerendt, I. K., Lawrence, A. D., & Brooks, D. J. (2004). Reward processing in health and Parkinson's disease: Neural organization and reorganization. Cerebral Cortex, 14, 73–80.
Goldman, W. P., Baty, J. D., Buckles, V. D., Sahrmann, S., & Morris, J. C. (1998). Cognitive and motor functioning in Parkinson disease: Subjects with and without questionable dementia. Archives of Neurology, 55, 674–680.
Hayes, A., Davidson, M., Keele, S. W., & Rafal, R. D. (1998). Toward a functional analysis of the basal ganglia. Journal of Cognitive Neuroscience, 10, 178–198.
Healy, A. F., Wohldmann, E. L., Sutton, E. M., & Bourne, L. E., Jr. (2006). Specificity effects in training and transfer of speeded responses. Journal of Experimental Psychology. Learning, Memory, and Cognition, 32, 534–546.
Hedden, T., & Gabrieli, J. D. (2010). Shared and selective neural correlates of inhibition, facilitation, and shifting processes during executive control. NeuroImage, 51(1), 421–431.
Helmich, R. C., Aarts, E., de Lange, F. P., Bloem, B. R., & Toni, I. (2009). Increased dependence of action selection on recent motor history in Parkinson's disease. Journal of Neuroscience, 29, 6105–6113.
Herzallah, M. M., Moustafa, A. A., Misk, A. J., Al-Dweib, L. H., Abdelrazeq, S. A., Myers, C. E., et al. (2010). Depression impairs learning whereas anticholinergics impair transfer generalization in Parkinson patients tested on dopaminergic medications. Cognitive and Behavioral Neurology, 23, 98–105.
Jaeggi, S. M., Buschkuehl, M., Jonides, J., & Perrig, W. J. (2008). Improving fluid intelligence with training on working memory. Proceedings of the National Academy of Sciences, 105, 6829–6833.
Joosten, J. P. A., Coenders, C. J. H., & Eling, P. A. T. M. (1995). Shifting behavior: An analysis of response patterns of Parkinson patients in discrimination learning. Brain and Cognition, 29, 115–126.
Karbach, J., & Kray, J. (2009). How useful is executive control training? Age differences in near and far transfer of task-switching training. Developmental Science, 12, 978–990.
Keys, B. A., & White, D. A. (2000). Exploring the relationship between age, executive abilities, and psychomotor speed. Journal of the International Neuropsychological Society, 6, 76–82.
Kish, S. J., Shannak, K., & Hornykiewicz, O. (1988). Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease Pathophysiologic and clinical implications. The New England Journal of Medicine, 318, 876–880.
Knowlton, B. J., Mangels, J. A., & Squire, L. R. (1996). A neostriatal habit learning system in humans. Science, 273, 1399–1402.
Kramer, A. F., Hahn, S., & Gopher, D. (1999). Task coordination ad aging: Explorations of executive control process in the task switching paradigm. Acta Psychologica, 101, 339–378.
Kray, J., & Lindenberger, U. (2000). Adult age differences in task switching. Psychology and Aging, 15, 126–147.
Kunig, G., Leenders, K. L., Martin-Solch, C., Missimer, J., Magyar, S., & Schultz, W. (2000). Reduced reward processing in the brains of Parkinsonian patients. NeuroReport, 11, 3681–3687.
Lee, E. Y., Cowan, N., Vogel, E. K., Rolan, T., Valle-Inclan, F., & Hackley, S. A. (2010). Visual working memory deficits in patients with Parkinson's disease are due to both reduced storage capacity and impaired ability to filter out irrelevant information. Brain, 133, 2677–2689.
Lees, A. J., & Smith, E. (1983). Cognitive deficits in the early stages of Parkinson's disease. Brain, 106, 257–270.
Locke, H. S., & Braver, T. S. (2008). Motivational influences on cognitive control: Behavior, brain activation, and individual differences. Cognitive, Affective, & Behavioral Neuroscience, 8, 99–112.
MacDonald, A. W., III, & Carter, C. S. (2002). Cognitive experimental approaches to investigating impaired cognition in schizophrenia: A paradigm shift. Journal of Clinical and Experimental Neuropsychology, 24, 873–882.
Maddox, W. T., Aparicio, P., Marchant, N. L., & Ivry, R. B. (2005). Rule-based category learning is impaired in patients with Parkinson's disease but not in patients with cerebellar disorders. Journal of Cognitive Neuroscience, 17, 707–723.
Maylor, E. A., & Lavie, N. (1998). The influence of perceptual load on age differences in selective attention. Psychology and Aging, 13, 563–573.
Mazzoni, P., Hristova, A., & Krakauer, J. W. (2007). Why don't we move faster? Parkinson's disease, movement vigor, and implicit motivation. Journal of Neuroscience, 27, 7105–7116.
Mimura, M., Oeda, R., & Kawamura, M. (2006). Impaired decision-making in Parkinson's disease. Parkinsonism & Related Disorders, 12, 169–175.
Monsell, S. (2003). Task switching. Trends in Cognitive Sciences, 7, 134–140.
O'Doherty, J., Dayan, P., Schultz, J., Deichmann, R., Friston, K., & Dolan, R. J. (2004). Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science, 304, 452–454.
Osman, M., Wilkinson, L., Beigi, M., Castaneda, C. S., & Jahanshahi, M. (2008). Patients with Parkinson's disease learn to control complex systems via procedural as well as non-procedural learning. Neuropsychologia, 46, 2355–2363.
Owen, A. M., Roberts, A. C., Hodges, J. R., Summers, B. A., Polkey, C. E., & Robbins, T. W. (1993). Contrasting mechanisms of impaired attentional set-shifting in patients with frontal lobe damage or Parkinson's disease. Brain, 116, 1159–1175.
Pessoa, L. (2009). How do emotion and motivation direct executive control? Trends in Cognitive Sciences, 13, 160–166.
Pizzagalli, D. A., Holmes, A. J., Dillon, D. G., Goetz, E. L., Birk, J. L., Bogdan, R., et al. (2009). Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. The American Journal of Psychiatry, 166, 702–710.
Pollux, P. M. J. (2004). Advance preparation of set-switches in Parkinson's disease. Neuropsychologia, 42, 912–918.
Ravizza, S. M., & Ciranni, M. A. (2002). Contributions of the prefrontal cortex and basal ganglia to set shifting. Journal of Cognitive Neuroscience, 14, 472–483.
Ravizza, S. M., & Ivry, R. B. (2001). Comparison of the basal ganglia and cerebellum in shifting attention. Journal of Cognitive Neuroscience, 13, 285–297.
Robbins, T. W., James, M., Owen, A. M., Sahakian, B. J., Lawrence, A. D., McInnes, L., et al. (1998). A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: Implications for theories of executive functioning and cognitive aging. Journal of the International Neuropsychological Society, 4, 474–490.
Rogers, R. D., Sahakian, B. J., Hodges, J. R., Polkey, C. E., Kennard, C., & Robbins, T. W. (1998). Dissociating executive mechanisms of task control following frontal lobe damage and Parkinson's disease. Brain, 121, 815–842.
Rutledge, R. B., Lazzaro, S. C., Lau, B., Myers, C. E., Gluck, M. A., & Glimcher, P. W. (2009). Dopaminergic drugs modulate learning rates and perseveration in Parkinson's patients in a dynamic foraging task. Journal of Neuroscience, 29, 15104–15114.
Santesso, D. L., Steele, K. T., Bogdan, R., Holmes, A. J., Deveney, C. M., Meites, T. M., et al. (2008). Enhanced negative feedback responses in remitted depression. NeuroReport, 19, 1045–1048.
Schonberg, T., Daw, N. D., Joel, D., & O'Doherty, J. P. (2007). Reinforcement learning signals in the human striatum distinguish learners from nonlearners during reward-based decision making. Journal of Neuroscience, 27, 12860–12867.
Schultz, W. (2007). Behavioral dopamine signals. Trends in Neurosciences, 30, 203–210.
Shohamy, D., Myers, C. E., Grossman, S., Sage, J., Gluck, M. A., & Poldrack, R. A. (2004). Cortico-striatal contributions to feedback-based learning: Converging data from neuroimaging and neuropsychology. Brain, 127, 851–859.
Slaughter, J. R., Slaughter, K. A., Nichols, D., Holmes, S. E., & Martens, M. P. (2001). Prevalence, clinical manifestations, etiology, and treatment of depression in Parkinson's disease. The Journal of Neuropsychiatry and Clinical Neurosciences, 13, 187–196.
Steele, J. D., Kumar, P., & Ebmeier, K. P. (2007). Blunted response to feedback information in depressive illness. Brain, 130, 2367–2374.
Sylvester, C.-Y. C., Wager, T. D., Lacey, S. C., Hernandez, L., Nichols, T. E., Smith, E. E., et al. (2003). Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia, 41, 357–370.
Taylor, A. E., Saint-Cyr, J. A., & Lang, A. E. (1986). Frontal lobe dysfunction in Parkinson's disease. Brain, 109, 845–883.
Taylor, S. F., Welsh, R. C., Wager, T. D., Phan, K. L., Fitzgerald, K. D., & Gehring, W. J. (2004). A functional neuroimaging study of motivation and executive function. NeuroImage, 21, 1045–1054.
Tricomi, E. M., Delgado, M. R., McCandliss, B. D., McClelland, J. L., & Fiez, J. A. (2006). Performance feedback drives caudate activation in a phonological learning task. Journal of Cognitive Neuroscience, 18, 1029–1043.
Tricomi, E. M., Delgado, M. R., & Fiez, J. A. (2004). Modulation of caudate activity by action contingency. Neuron, 41, 281–292.
van den Wildenberg, W. P., van Boxtel, G. J., van der Molen, M. W., Bosch, D. A., Speelman, J. D., & Brunia, C. H. (2006). Stimulation of the subthalamic region facilitates the selection and inhibition of motor responses in Parkinson's disease. Journal of Cognitive Neuroscience, 18, 626–636.
Wachter, T., Lungu, O. V., Liu, T., Willingham, D. T., & Ashe, J. (2009). Differential effect of reward and punishment on procedural learning. Journal of Neuroscience, 29, 436–443.
Werheid, K., Koch, I., Reichert, K., & Brass, M. (2007). Impaired self-initiated task preparation during task switching in Parkinson's disease. Neuropsychologia, 45, 273–281.
Witt, K., Daniels, C., Daniel, V., Schmitt-Eliassen, J., Volkmann, J., & Deuschl, G. (2006). Patients with Parkinson's disease learn to control complex systems—an indication for intact implicit cognitive skill learning. Neuropsychologia, 44, 2445–2451.
Wright, L. L., & Elias, J. W. (1979). Age differences in the effects of perceptual noise. Journal of Gerontology, 34, 704–708.
Wylie, S. A., van den Wildenberg, W. P., Ridderinkhof, K. R., Bashore, T. R., Powell, V. D., Manning, C. A., et al. (2009). The effect of Parkinson's disease on interference control during action selection. Neuropsychologia, 47, 145–157.
Acknowledgements
We would like to thank Denise VanEtten for her help in recruiting participants and Stephanie Oppenheim for testing participants. Thanks also to Elizabeth Tricomi and Tim Pleskac for their insightful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ravizza, S.M., Goudreau, J., Delgado, M.R. et al. Executive function in Parkinson’s disease: contributions of the dorsal frontostriatal pathways to action and motivation. Cogn Affect Behav Neurosci 12, 193–206 (2012). https://doi.org/10.3758/s13415-011-0066-6
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-011-0066-6