Article Text
Abstract
Objectives: The cytokine interleukin (IL)-1 mediates ischaemic brain damage in rodents. The endogenous, highly selective, IL-1 receptor antagonist (IL-1ra) protects against ischaemic cerebral injury in a range of experimental settings, and IL-1ra causes a marked reduction of cell death when administered peripherally or at a delay in transient cerebral ischaemia. We report here the first randomised, double blind, placebo controlled trial of recombinant human IL-1ra (rhIL-1ra) in patients with acute stroke.
Methods: Patients within 6 hours of the onset of symptoms of acute stroke were randomised to rhIL-1ra or matching placebo. Test treatment was administered intravenously by a 100 mg loading dose over 60 seconds, followed by a 2 mg/kg/h infusion over 72 h. Adverse events and serious adverse events were recorded for up to 3 months, serial blood samples were collected for biological markers up to 3 months, and 5–7 day brain infarct volume was measured by computed tomography.
Results: No adverse events were attributed to study treatment among 34 patients randomised. Markers of biological activity, including neutrophil and total white cell counts, C reactive protein, and IL-6 concentrations, were lower in rhIL-1ra treated patients. Among patients with cortical infarcts, clinical outcomes at 3 months in the rhIL-1ra treated group were better than in placebo treated.
Conclusions: These data suggest that rhIL-1ra is safe and well tolerated in acute stroke. In addition, rhIL-1ra exhibited biological activity that is relevant to the pathophysiology and clinical outcome of ischaemic stroke. Our findings identify rhIL-1ra as a potential new therapeutic agent for acute stroke.
- BI, Barthel Index
- ESR, erythrocyte sedimentation rate
- CRP, C reactive protein
- CT, computed tomography
- IL, interleukin
- NIHSS, National Institutes of Health Stroke Scale
- OCSP, Oxfordshire Community Stroke Project
- PICH, primary intracerebral haemorrhage
- rhIL-1ra, recombinant human interleukin-1 receptor antagonist
- SAE, serious adverse event
- TACS, total anterior circulation syndromes
- stroke
- acute
- inflammation
- cytokines
- interleukin-1 receptor antagonist
Statistics from Altmetric.com
- BI, Barthel Index
- ESR, erythrocyte sedimentation rate
- CRP, C reactive protein
- CT, computed tomography
- IL, interleukin
- NIHSS, National Institutes of Health Stroke Scale
- OCSP, Oxfordshire Community Stroke Project
- PICH, primary intracerebral haemorrhage
- rhIL-1ra, recombinant human interleukin-1 receptor antagonist
- SAE, serious adverse event
- TACS, total anterior circulation syndromes
Endogenous interleukin-1 (IL)-1 has been implicated in acute experimental cerebral ischaemia using several complementary approaches.1 IL-1 receptor antagonist (IL-1ra) is the naturally occurring, highly selective, competitive antagonist that blocks all known actions of IL-1, but that has, to date, no other known actions.2 Intracerebroventricular or peripheral administration of IL-1ra significantly reduces neuronal injury experimentally induced by transient or permanent middle cerebral artery occlusion, global ischaemia, perinatal hypoxia/ischaemia, or by excitotoxic, traumatic, haemorrhagic, or heat stroke damage.1,3 Crucially, neuroprotection is maintained when IL-1ra is administered 3 hours after temporary middle cerebral artery occlusion,3 and 8–12 hours after global ischaemia or brain trauma.4 It is also effective when administered in high doses peripherally.4 In addition to reduced lesion size, IL-1ra also increases neuronal survival, reduces oedema, glial activation, and invasion of peripheral immune cells, and improves behavioural outcome.1 An active transport mechanism has been described for IL-1ra that allows transfer into the brain,5 and the disrupted integrity of the blood–brain barrier in the injured brain might further improve brain access. It is also possible that IL-1ra exerts some actions outside the brain. In summary, IL-1ra limits injury in all forms of cerebral ischaemia tested to date, and the preclinical data for IL-1ra are probably more extensive than for any other potential neuroprotective agent that has entered the clinical arena.
Extensive toxicity studies have revealed no major adverse effects of recombinant human IL-1ra (rhIL-1ra) in animals or humans. Subcutaneous rhIL-1ra is safe and effective in rheumatoid arthritis patients.6 Intravenous rhIL-1ra has also been investigated in severe sepsis; adverse event profiles in the treatment groups (active and placebo) were comparable, and no serious adverse events (SAEs) could be attributed to rhIL-1ra.7
We have undertaken the first randomised, double blind, placebo controlled study of rhIL-1ra in acute stroke patients, with the primary aim of assessing safety. Secondary aims were to obtain information on biological activity and efficacy, based on 3 month survival, well validated stroke scales, a range of biological markers, and computed tomography (CT) cerebral infarct volume.
METHODS
Hypothesis, location, and selection of patients
The primary hypothesis tested was that intravenous rhIL-1ra is safe and well tolerated in acute stroke patients. Secondary objectives were to test whether rhIL-1ra has biological activity, and to perform an exploratory analysis of efficacy. The trial was conducted at an acute teaching hospital trust in the UK, and received appropriate ethics approval. Inclusion criteria were (a) age ⩾18 years, (b) written informed consent/assent, and (c) within 6 hours of onset of symptoms of acute stroke. Exclusion criteria were (a) clinically significant concurrent medical condition affecting evaluation of tolerability, safety or efficacy, (b) rapid clinical improvement, (c) National Institutes of Health Stroke Scale (NIHSS) score ⩽4, (a) pre-stroke modified Rankin score (MRS) ⩾4, (d) previous inclusion in the current study, (e) investigational drug or device within the previous 30 days, and (f) pregnancy or breast feeding.
Randomisation, treatment, and study procedures
Initial assessment included NIHSS score8 and MRS9 prior to randomisation. Aural temperature, Barthel Index (BI)10 and Oxfordshire Community Stroke Project (OCSP) classification11 were also recorded. Treatment group assignment (rhIL-1ra or matching placebo) was performed by an independent, interactive voice response service (BioCall, Nottingham, UK). Restricted block randomisation balanced the groups for age (<70 and ⩾70 years), baseline severity (NIHSS score 4–9, 10–20, ⩾21), and time since onset (<4, ⩾4 hours). The facility for emergency unblinding existed, but was not used. Test treatment was initiated as soon as possible. Computed tomography (CT) brain scans were performed within 24 hours of admission and at 5–7 days in those patients with ischaemic stroke for determination of cerebral infarct volume.12 Anakinra (Kineret®; Amgen, Thousand Oaks, CA, USA) is the recombinant methionylated form of hIL-1ra, referred to as rhIL-1ra here. Intravenous test treatment administration was by 100 mg loading dose over 60 seconds, followed by consecutive 2 mg/kg/h infusions over 72 hours.
Blood samples and measurement of markers of biological activity and IL-1ra
Venous blood samples were taken immediately prior to bolus administration (baseline), the next 0900 time point where admission was before 0700 or after 1100, 24 hours after admission, then on days 2, 3, 4, 5–7, and 3 months at 0900. Samples were collected into serum gel, fluoride oxalate/EDTA, and EDTA containing tubes (Sarstedt, UK) for serum biochemical profile and plasma glucose (Roche Integra 700 analyser), erythrocyte sedimentation rate (ESR) (StaRRsed III analyser), and full blood and differential white blood cell counts (Coulter Gen-S analyser). Blood sample collection, processing, C reactive protein (CRP), and IL-6 measurements by ELISA were as described previously.13 A similar method was used to measure plasma IL-1ra, which was captured using a monoclonal antibody (catalogue no. 58.118.08; Biosource, Nivelles, Belgium) and secondarily bound using a biotinylated monoclonal antibody (catalogue no. 58.118.02; Biosource). Detection employed streptavidin conjugated horseradish peroxidase (Zymed), developed with orthophenylene diamine (Sigma, Poole, Dorset, UK). Standard IL-1ra was calibrated against the international standard human IL-1ra (92/644; National Institute for Biological Standards and Control, South Mimms, UK). Minimum sensitivities were as follows: IL-6, 6 pg/ml; CRP, 25 μg/l; IL-1ra (without rhIL-1ra treatment), 15 pg/ml; IL-1ra (with rhIL-1ra treatment), 50 pg/ml. Interassay coefficients of variation, determined in the appropriate working range, were 15% at 43.4 pg/ml to 9% at 373 pg/ml (IL-6); 22% at 430 μg/l to 21% at 25.4 mg/l (CRP), 16% at 139 pg/ml to 7% at 1220 pg/ml (IL-1ra without rhIL-1ra treatment), and 25% at 250 pg/ml to 4% at 8537 pg/ml (IL-1ra with rhIL-1ra treatment).
Outcome measures and definitions of adverse events
Primary outcome measures were, in the first 72 hours after the initiation of test treatment, (a) SAEs and (b) increase in NIHSS score >4 points. Secondary outcome measures were adverse events, markers of biological activity (including WBC count, ESR, CRP, and IL-6), CT brain infarct volume at 5–7 days, and clinical outcomes (survival to 3 months, NIHSS, BI and MRS scores at 3 months). A non-serious adverse event was defined as any undesirable medical experience occurring to a patient whether or not considered related to the test treatment, not including abnormal laboratory values without clinical consequences.14 An SAE was defined as an event suggesting a significant hazard or side effect, regardless of the investigators’ opinion on the relationship to test treatment, including, but not limited to, any event that (a) was fatal, (b) was life threatening (places the patient at immediate risk of death), (c) required inpatient hospitalisation or prolongation of existing hospitalisation, (d) was a persistent or significant disability/incapacity, or (e) was a congenital anomaly/birth defect.15 AEs and SAEs were recorded to 3 months and reported to an independent data monitoring committee and the appropriate regulatory agencies. They were later classified by organ system class using the Medical dictionary for regulatory activities.16 All outcome data were measured by the clinical investigators.
Sample size considerations and statistical analysis
Our aim was to recruit 50 patients per group, in order to have 40 cortical infarcts per group. This number was deemed sufficient to rule out common, unanticipated adverse events in acute stroke and provide pilot data for planning phase III trials of efficacy. Recruitment fell short of this target for practical reasons unrelated to study results, so the presented data provide a valid comparison. Analysis of clinical outcome was pre-specified to be undertaken in patients with cortical infarcts only, in order to compare a more homogenous group. Furthermore, animal studies have shown that rhIL-1ra is most protective against cortical injury.3 Cortical stroke was defined by clinical classification, and on the basis of CT evidence, as primary intracerebral haemorrhage (PICH) or infarction. Clinical classification alone was used if no lesion was seen on CT. Analysis of all data, for both the complete sample and for the pre-specified subgroup of cortical infarct patients, was planned to follow the intention to treat principle. In practice, all patients received treatments as allocated. Analysis of serious adverse events and deterioration in NIHSS score used Fisher’s exact test. An increase in NIHSS score of more than four points as a primary outcome measure was chosen arbitrarily, but a four point change is widely accepted to represent an important change in the degree of neurological impairment.17 CT infarct volume at 5–7 days was compared by 95% confidence interval for the difference in group means, and survival to 3 months was compared using the Kaplan-Meier method with log rank test. All efficacy analysis was secondary and exploratory.
RESULTS
Treatment assignment, patient characteristics, and rhIL-1ra infusion kinetics
Of 218 patients screened between February 2001 and July 2003, 184 (84%) were excluded (fig 1). Thus, 34 patients were randomised, 17 in each group. Median age (72 years), baseline NIHSS score (19), time since onset (3.6 hours), pre-stroke disability, stroke severity/subtype, and risk factor profile were similar in the two groups (table 1). All patients were treated within 6.25 hours, and 19 (56%) within 4 hours of onset of symptoms. Overall, 29 (85%) patients had ischaemic stroke, and 24 had cortical infarcts (71%). Of 17 patients who received rhIL-1ra, 12 (71%) had ischaemic stroke and 10 (59%) had cortical infarcts (table 2). All five patients (29%) with PICH were in the active group. Patients in the two groups received similar standard treatments during the study, including antiplatelet, anticoagulant, and antihypertensive agents and statins, where appropriate, and in accordance with stroke subtype. No patients were treated with thrombolytic agents.
Demography and baseline characteristics
Serious adverse events (SAEs) and non-serious adverse events (AEs)
Trial profile. *Reasons for exclusion were: duration of symptoms >6 hours or time of onset not reliably known (n = 66), mild stroke (NIHSS score ⩽4) or rapid clinical improvement (n = 32), suspected non-stroke diagnosis (n = 29), no consent or assent (n = 15), significant concurrent medical condition (n = 15), significant pre-existing disability (MRS ⩾4) (n = 11), and others (n = 16).
The test treatment was very well tolerated, being completed in 29 (85%) of patients (fig 1), with a median (interquartile range, IQR) duration of 72.3 hours (72.1 to 72.6 hours). During the test treatment infusion, the median (IQR) IL-1ra plasma concentration was between 370 pg/ml (302 to 561 pg/ml) and 617 pg/ml (451 to 705 pg/ml) in the patients receiving placebo, and between 28 μg/ml (21 to 38 μg/ml) and 30 μg/ml (25 to 36 μg/ml) in those receiving rhIL-1ra (supplementary fig. 1, available on the journal website: http://www.jnnp.com/supplemental).
Safety analysis
In the first 72 hours there were three SAEs (all deaths) in three patients in the placebo group and two SAEs in two patients in the active group (one death, one neurological deterioration secondary to PICH) (p = 1.0) (table 2). None was attributed to test treatment. No other patients experienced an increase of more than four points in NIHSS score. There were 14 non-serious AEs in 10 patients in the placebo group and 9 non-serious AEs in 9 patients in the active group by 72 hours (p = 1.0). Again, none was attributed to test treatment.
By 3 months, five SAEs had occurred in each group: four deaths and one recurrent stroke in the placebo group (in five patients), and three deaths, one neurological deterioration secondary to PICH, and one recurrent stroke in the active group (five patients) (p = 1.0). All deaths were certified as being due to the index stroke, and the death rate was similar between the two groups (p = 0.65). Other than the seven deaths, no other patients experienced an increase of more than four points in NIHSS score. Patient survival to 3 months was similar in the two groups. There were 31 non-serious adverse events in both the placebo group (in 12 patients) and in the active group (in 13 patients) by 3 months (p = 1.0). Again, none was attributed to test treatment.
The most frequently occurring adverse events were infections. None of these was classified as an SAE. In all patients, there were 25 infectious episodes, representing 35% of all adverse events (including non-serious AEs and SAEs). Nine infectious episodes occurred in the placebo group and 16 in the active group, representing 25% and 44% of all adverse events respectively. Fewer infections were seen in patients with cortical infarcts, with nine events in the placebo group (28% of all adverse events) and six in the active group (40% of all adverse events). Eight infectious episodes were seen in the five patients with PICH in the active group (50% of all adverse events). No emergent changes of clinical significance for the test treatment were observed in any haematological or biochemical laboratory data.
Analysis of biological activity
Peripheral total white blood cell counts and neutrophil counts were lower in the rhIL-1ra-treated compared with placebo treated patients after the initiation of test treatment (fig 2A,B). During test treatment infusion, median total white blood cell count was up to 27% lower, and median neutrophil count up to 45% lower in the rhIL-1ra compared with placebo treated patients. Similar trends were observed with plasma CRP and IL-6 concentrations (fig 2 C,D). Mean log CRP concentration was up to 28% lower, and mean log IL-6 concentration up to 17% lower in the rhIL-1ra compared with placebo treated patients. Individual profile plots of neutrophil count, CRP and IL-6 are shown in supplementary fig 2 (available on the journal website: http://www.jnnp.com/supplemental). No differences between the groups were seen in ESR or aural temperature (supplementary fig 3; available on the journal website: http://www.jnnp.com/supplemental). CT brain infarct volume was similar in the groups (p = 0.6), mean difference (95% confidence interval) 4.5 cm3 (−61.7 to 70.7) (supplementary table A; available on the journal website; http://www.jnnp.com/supplemental).
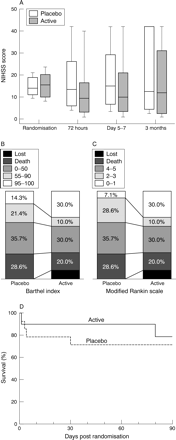
Biological markers: (A) neutrophil count; (B) total white cell count; (C) CRP concentration; (D) interleukin-6 concentration. In each of A–D, data are shown as mean (with unidirectional 95% confidence intervals).
Clinical outcome in patients with cortical infarcts. (A) Boxplot of NIHSS score (boxes denote medians and interquartile ranges; whiskers denote 5th and 95th centiles); (B) Barthel index at 3 months; (C) modified Rankin scale at 3 months; (D) Kaplan-Meier survival curves showing cumulative survival (%) to 3 months by treatment group.
Exploratory efficacy analysis in subjects with cortical infarcts
Baseline NIHSS and pre-stroke BI and MRS scores for all patients are shown in table 1 (for secondary outcome data for all patients see supplementary table A; available on the journal website; http://www.jnnp.com/supplemental). In patients with cortical infarcts, median baseline NIHSS score (IQR) was 14 (11 to 19) and 16 (10 to 20) in the placebo and active groups respectively; pre-stroke median BI (IQR) was 100 (100 to 100) in both groups, and pre-stroke median MRS (IQR) was 1 (0 to 1) and 0 (0 to 0) in the placebo and active groups respectively. NIHSS scores were lower in the rhIL-1ra-treated group compared with placebo at 72 hours, and at day 5 to 7, in patients with cortical infarcts (fig 3A). In patients with cortical infarcts, there was a median reduction of four points in NIHSS score at 3 months in rhIL-1ra treated patients, compared with a change of one point in those receiving placebo. At 3 months, a greater proportion of patients receiving rhIL-1ra (30%) had a BI of 95–100 than those receiving placebo (14%) (fig 3B). Similarly, 30% of patients receiving rhIL-1ra had a modified Rankin scale of 0–1 at 3 months compared with 7% of those receiving placebo (fig 3C). A BI of 95–100 or a MRS score of 0–1 both represent favourable outcomes with minimal or no disability. Survival to 3 months is shown in fig 3D.
DISCUSSION
We provide evidence to suggest that intravenous rhIL-1ra is safe and well tolerated in patients with acute stroke. Biological activity is suggested by a reduction in total white cell count, neutrophil count, and plasma CRP and IL-6 concentrations in patients receiving rhIL-1ra compared with placebo. Exploratory efficacy analysis indicates a greater proportion of patients receiving rhIL-1ra with minimal or no disability at 3 months compared with placebo.
The single centre study design gave us the opportunity to conduct a careful, exploratory investigation of biological activity and efficacy in addition to the detailed evaluation of safety. Although the time window of 6 hours used here is short, experimental models suggest that benefit will be seen in acute stroke only within a few hours of the onset of cerebral ischaemia. Given the demonstrated benefit of rhIL-1ra for at least 3 hours after initiation of experimental cerebral ischaemia,3 the median (interquartile range) interval to treatment here of 3.6 hours (2.8 to 5.0) is biologically appropriate.
Administration over 72 hours was based on the profile of biologically active IL-1 in experimental stroke models and the time course of emerging injury in patients with acute stroke.1,18 Continuous infusion was required, owing to the very short half life of rhIL-1ra (approx. 3 mins) in plasma.19 The dose regimen of 100 mg bolus followed by 2 mg/kg/h infusion for 72 hours was chosen as this is the largest known to have been tolerated previously,7 and this regimen provided mean (SD) plasma levels of IL-1ra of 25 (13) μg/ml,20 very similar to those seen in the present study. Importantly, these plasma concentrations are significantly higher than those achieved in rat models (⩾100 ng/ml) where rhIL-1ra was an effective treatment.21 In a study examining the kinetics of IL-1ra, we found that on admission patients with stroke had slightly elevated plasma IL-1ra concentrations(unpublished observations). In contrast to a previous study,22 we did not find that IL-1ra increased during the first week. However, the plasma IL-1ra concentration achieved in the patients receiving rhIL-1ra is approximately 100 000 times greater than that in patients receiving placebo.
Because the primary aim of this study was to assess safety, it was considered essential to include patients with both ischaemic and primary haemorrhagic strokes. While CT was performed in all patients within 24 hours of admission, this did not inform the protocol. The excess of patients with PICH in the active group arose by chance; however, no specific safety concerns were identified in these patients. Although cortical infarcts are more likely to benefit than other stroke subtypes from neuroprotection,23 there is evidence of an inflammatory reaction around intracerebral haemorrhage (ICH).24 Furthermore, although it is the subject of debate, there is some evidence of an ischaemic penumbra in PICH.25 In an animal model, adenovirus mediated IL-1ra overexpression attenuated ICH induced brain oedema formation,26 and rhIL-1ra is neuroprotective in animal models of brain trauma in which haemorrhage is a significant component of injury.27 Thus rhIL-1ra may have beneficial effects in ICH.
An apparent anomaly was observed between the OCSP classification and the median baseline NIHSS score. Despite the greater proportion of patients with total anterior circulation syndromes (TACS) in the placebo group (59%) compared with the active group (29%), the median NIHSS score at baseline among patients with cortical infarcts was greater in the active group (16) than in the placebo group (14). This appears to be largely explained by a preponderance of right hemisphere TACS (70%) in the placebo group compared with the active group (20%), as the NIHSS is known to favour left hemisphere strokes.28
These data are especially important because rhIL-1ra is a potential neuroprotective agent for stroke that has demonstrated biological activity relevant to the disease process and its clinical outcome. Inflammation is likely to predispose to stroke,29 and cellular inflammation is important in the pathology of acute cerebral ischaemia,30 while white blood cell counts are elevated early after stroke13 and are associated with poor clinical outcome.31 Reduced total white blood cell and neutrophil counts in patients receiving rhIL-1ra in this trial are therefore likely to have pathophysiological importance in stroke. High circulating levels of inflammatory markers (which may reflect local tissue levels of IL-1), in particular IL-6 and CRP, are predictive of poor clinical outcome,32 and therefore the effects on plasma IL-6 and CRP concentrations in rhIL-1ra-treated patients are also likely to be relevant. The absence of any significant effect on aural temperature is not altogether surprising because pyrogenic and neurotoxic effects of IL-1β can be dissociated,33 and rhIL-1ra does not fully attenuate the temperature increase seen in experimental cerebral ischaemia (Rothwell, unpublished data), both observations supporting the argument that the mechanisms of neuroprotection by rhIL-1ra extend beyond its antipyretic effects.
IL-1 has an important role in host defence against infection.2 rhIL-1ra has been associated with an increased incidence of serious infections (2%) versus placebo (1%) when administered over 6 months to patients predisposed to infection either because of immunosuppressive therapy or underlying disease (data provided by Amgen). Infection was the commonest system organ class of adverse events in this study, representing 35% of adverse events overall; 25% of those in placebo treated and 44% of those in patients receiving rhIL-1ra. Among patients with cortical infarcts, infections constituted 28% and 40% of all adverse events in the placebo and active groups respectively. The differences in these proportions are largely explained by the higher rate of infections (50% of all adverse events) in patients with PICH receiving rhIL-1ra. Whether PICH per se renders patients more prone to infectious complications has not, to our knowledge, been the subject of any previous systematic study. The infectious episodes amongst all patients were predominantly bacterial and typical of infections often seen in patients with acute stroke,34 rather than atypical, opportunistic, fungal, or viral infections, and none was classified as serious. Despite the lower neutrophil count in rhIL-1ra treated patients, neutropenia (neutrophil count <1×109/l) did not occur in any patient during this study.
All measures of clinical outcome were more favourable in the rhIL-1ra treated group, but these analyses were secondary and exploratory. However, we cannot rule out potential confounding effects such as the greater proportion of patients with TACS (who might be expected to have a worse outcome) in the placebo group (59%) than in the active group (29%) as an influence. It is, however, encouraging that 3 month scores on the BI and modified Rankin scale consistent with minimal or no disability were recorded in a larger proportion of rhIL-1ra than placebo treated patients, and that this difference was even greater in the patients with cortical infarcts, an effect that was predicted from pre-clinical studies.23
These data show that in this small sample rhIL-1ra was well tolerated and appeared to be safe when administered within 6 hours of acute stroke. In addition, rhIL-1ra exhibited biological activity in these patients that is of relevance to the pathophysiology and clinical outcome of stroke. Before consideration of a phase III study of rhIL-1ra, more information about its pharmacokinetics, particularly of entry into the brain, is required, and possibly a dose ranging study to ascertain the most effective dose. Careful consideration of patient selection criteria and outcome measures will be required in planning an eventual efficacy study.
Acknowledgments
This study was funded by a grant from Research into Ageing, provided by the UK Community Fund, and supported by Salford Royal Hospitals National Health Service (NHS) Trust Research and Development Directorate. H C A Emsley is funded by the University of Liverpool, C J Smith is funded by North Manchester Healthcare NHS Trust, A Vail and S J Hopkins are funded by Salford Royal Hospitals NHS Trust, N J Rothwell is supported by the Medical Research Council and R F Georgiou and P J Tyrrell are funded by the University of Manchester. We would like to thank the late Michael Traub, Amgen, for his helpful discussions and for organising the supply of test treatment. We are also grateful to the various clinical departments at Salford Royal Hospitals NHS Trust who provided assistance.
REFERENCES
Supplementary materials
Files in this Data Supplement:
Footnotes
-
The IL-1RA in Acute Stroke Investigators are as follows. Steering committee: P J Tyrrell (UK), NJ Rothwell (UK), S J Hopkins (UK), A Vail (UK), G J del Zoppo (USA), J M Hallenbeck (USA); Data Monitoring committee: G Ford (UK), A Sharma (UK), S Hollis (UK); study investigators (UK) P J Tyrrell, N J Rothwell, S J Hopkins, A Vail, H C A Emsley, C J Smith, R F Georgiou, C M Gavin, E M Barberan, A Parry-Jones, J Selvarajah, J Staniland, C Sherrington, D G Hughes, IW Turnbull, G Morris, S Scarth, K Illingworth.
-
Competing interests: N J Rothwell holds a patent for interleukin-1 receptor antagonist in acute neurodegeneration.