Abstract
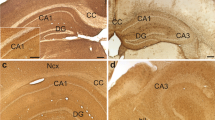
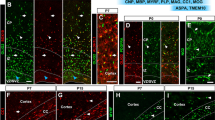
We have used sections of adult mouse brain to determine whether antibodies specific for oligodendroglia (anti-carbonic anhydrase II, CA II; anti-galactocerebroside, GC; anti-myelin basic protein, MBP) and astroglia (anti-glial fibrillary acidic protein, GFAP; anti-S 100 protein) are suitable for quantitative studies of the proliferation and subsequent differentiation of these cells. Unlesioned adult mice received a single injection of 3H-thymidine (TdR) and were killed between 1 h and 70 days later. Quantitative evaluations of autoradiographs of 2-μm-thick serial sections stained immunocytochemically with the antibodies mentioned above or with Richardson's method for histological control led to the following conclusions. Anti-GC and anti-MBP stained only the oligodendrocytic processes and, thus, cannot be used in well-myelinated brain areas. Anti-CA II stained only a portion of the differentiated oligodendrocytes, but no proliferating cells. Anti-S 100 protein recognized all the astrocytes, but also many (interfascicular) oligodendrocytes. Anti-GFAP stained only a few astrocytes in the unlesioned mouse: all astrocytes may become GFAP-immunopositive only after wounding the brain. Thus, in contrast to in vitro studies, immunocytochemical studies with these antibodies on sections of adult animals cannot be recommended for the quantitative analysis of cell proliferation. In addition, our results show that differentiated glial cells proliferate in adult mice. Astro- and oligodendrocytes divide with the same cell cycle parameters and mode of proliferation up to about 1 month after 3H-TdR injection. In contrast to oligodendrocytes, some astrocytes might re-enter the cycle after a few weeks of quiescence.
Similar content being viewed by others
References
Abney ER, Bartlett PP, Raff MC (1981) Astrocytes, ependymal cells, and oligodendrocytes develop on schedule in dissociated cell cultures of embryonic rat brain. Dev Biol 83:301–310
Arenella LS, Herndon RM (1984) Mature oligodendrocytes. Division following experimental demyelination in adult animals. Arch Neurol 41:1162–1165
Bennett HS, Wyrick AD, Lee SW, McNeal JH Jr(1976) Science and art in preparing tissues embedded in plastic for light microscopy, with special reference to glycol methacrylate, glass knifes and simple stains. Stain Technol 51:71–97
Bologa L, Bisconte JC, Joubert R, Margules S, Herschkowitz N (1983) Proliferative activity and characteristics of immunocytochemically identified oligodendrocytes in embryonic mouse brain cell cultures. Exp Brain Res 50:84–90
Bologa-Sandru L, Siegrist HP, Z'Graggen A, Hofmann K, Wiesmann U, Dahl D, Herschkowitz N (1981) Expression of antigenic markers during the development of oligodendrocytes in mouse brain cell cultures. Brain Res 210:217–229
Cavanagh JB, Lewis PD (1969) Perfusion-fixation, colchicine and mitotic activity in the rat brain. J Anat 104:341–350
Delaunoy JP, Hog F, Devilliers G, Bansart M, Mandel P, Sensenbrenner M (1980) Developmental changes and localization of carbonic anhydrase in cerebral hemispheres of the rat and in rat glial cell cultures. Cell Mol Biol 26:235–240
Delaunoy JP, Langui D, Ghandour S, Labourdette G, Sensenbrenner M (1988) Influence of basic fibroblast growth factor on carbonic anhydrase expression by rat glial cells in primary culture. Int J Dev Neurosci 6:129–136
Eng LF (1980) The glial fibrillary acidic (GFA) protein. In: Bradshaw RA, Schneider DM (eds) Proteins of the nervous system, 2nd edn. Raven Press, New York, pp 85–118
Faddis BT, Vijayan VK (1988) Application of glial fibrillary acidic protein immunohistochemistry in the quantification of astrocytes in the rat brain. Am J Anat 183:316–322
ffrench-Constant C, Raff MC (1986) Proliferating bipotential glial progenitor cells in adult rat optic nerve. Nature 319:499–502
Ghandour MS, Skoff RP (1988) Expression of glactocerebroside in developing normal and jimpy oligodendrocytes in situ. J Neurocytol 17:485–498
Ghandour MS, Skoff RP (1991) Double-labeling in situ hybridization analysis of mRNAs for carbonic anhydrase II and myelin basic protein: expression in developing cultured glial cells. Glia 4:1–10
Ghandour MS, Langley OK, Vincendon G, Gombos G, Filippi D, Limozin N, Dalmasso C, Laurent G (1980) Immunochemical and immunohistochemical study of carbonic anhydrase II in adult rat cerebellum: a marker for oligodendrocytes. Neuroscience 5:559–571
Ghandour MS, Labourdette G, Vincendon G, Gombos G (1981) A biochemical and immunohistological study of S 100 protein in developing rat cerebellum. Dev Neurosci 4:98–109
Griffin R, Illis LS, Mitchell J (1972) Identification of neuroglia by light and electron microscopy. Acta Neuropathol 22:7–12
Hardy R, Reynolds R (1991) Proliferation and differentiation potential of rat forebrain oligodendroglial progenitors both in vitro and in vivo. Development 111:1061–1080
Hirayama M, Eccleston PA, Silberberg DH (1984) The mitotic history and radiosensitivity of developing oligodendrocytes in vitro. Dev Biol 104:413–420
Iwanaga T, Takahashi Y, Fujita T (1989) Immunohistochemistry of neuron-specific and glia-specific proteins. Arch Histol Cytol [Suppl] 52:13–24
Janeczko K (1989) Spatiotemporal patterns of the astroglial proliferation in rat brain injured at the postmitotic stage of postnatal development: a combined immunocytochemical and autoradiographic study. Brain Res 485:236–243
Janeczko K (1991) The proliferative response of S-100 protein-positive glial cells to injury in the neonatal rat brain. Brain Res 564:86–90
Kitamura T, Nakanishi K, Watanabe S, Endo Y, Fujita S (1987) GFA-protein gene expression on the astroglia in cow and rat brains. Brain Res 423:189–195
Korr H (1978 a) Autoradiographische Untersuchungen zur Proliferation verschiedener Zellelemente im Gehirn von Nagern während der prä- und postnatalen Ontogenese. Habilitations-schrift, University of Würzburg
Korr H (1978b) Combination of metallic impregnation and autoradiography of brain sections. A method for differentiation of proliferating glial cells in the brain of adult rats and mice. Histochemistry 59:111–116
Korr H (1980) Proliferation of different cell types in the brain. Adv Anat Embryol Cell Biol 61:1–72
Korr H (1981) Light microscopical autoradiography of nervous tissue. In: Heym C, Forssmann WG (eds) Techniques in neuroanatomical research. Springer, Berlin Heidelberg New York, pp 218–244
Korr H (1982) Proliferation of different cell types in the brain of senile mice. Autoradiographic studies with 3H- and 14C-thymidine. Exp Brain Res [Suppl] 5:51–57
Korr H (1986) Proliferation and cell cycle parameters of astrocytes. In: Fedoroff S, Vernadakis A (eds) Astrocytes. Cell biology and pathology of astrocytes, vol 3. Academic Press, Orlando, Florida, pp 77–127
Korr H, Schultze B, Maurer W (1973) Autoradiographic investigations of glial proliferation in the brain of adult mice. I. The DNA synthesis phase of neuroglia and endothelial cells. J Comp Neurol 150:169–176
Korr H, Koeser K, Oldenkott S, Schmidt H, Schultze B (1989) X-ray dose-effect relationship on unscheduled DNA synthesis and spontaneous unscheduled DNA synthesis in mouse brain cells studied in vivo. Radiat Environ Biophys 28:13–26
Korr H, Siewert E, Bertram C, Labourdette G, Sensenbrenner M (1992) Autoradiographic studies of rat astroglial cell proliferation in vitro with and without treatment with basic fibroblast growth factor. Cell Prolif 25:605–622
Labourdette G, Marks A (1975) Synthesis of S-100 protein in monolayer cultures of rat-glial cells. Eur J Biochem 58:73–79
Latov N, Nilaver G, Zimmerman EA, Johnson WG, Silverman AJ, Defendini R, Cote L (1979) Fibrillary astrocytes proliferate in response to brain injury. A study combining immunoperoxidase technique for glial fibrillary acidic protein and radioautography of tritiated thymidine. Dev Biol 72:381–384
Lillie RD, Fullmer HM (1976) Histopathologic technic and practical histochemistry, 4th edn. McGraw-Hill, New York
Ling EA, Paterson JA, Privat A, Mori S, Leblond CP (1973) Investigation of glial cells in semithin sections. I. Identification of glial cells in the brain of young rats. J Comp Neurol 149:43–72
Ludwin SK, Bakker DA (1988) Can oligodendrocytes attached to myelin proliferate? J Neurosci 8:1239–1244
Ludwin SK, Kosek JC, Eng LF (1976) The topographical distribution of S-100 and GFA proteins in the adult rat brain:an immunhistochemical study using horseradish peroxidase-labelled antibodies. J Comp Neurol 165:197–208
May B (1991) Autoradiographische Untersuchungen mit 3H-Thymidin zum Einfluß einer Röntgenbestrahlung des Kopfes bzw. einer Chloralhydratnarkose auf das Proliferationsverhalten von Zellen der subependymalen Schicht sowie Glia- und Endothelzellen im Gehirn der adulten Maus. Dissertation, Medizinische Fakultät, RWTH Aachen
McCarthy GF, Leblond CP (1988) Radioautographic evidence for slow astrocyte turnover and modest oligodendrocyte production in the corpus callosum of adult mice infused with 3H-thymidine. J Comp Neurol 271:589–603
Mori S, Leblond CP (1969a) Identification of microglia in light and electron microscopy. J Comp. Neurol 135:57–80
Mori S, Leblond CP (1969b) Electron microscopic features and proliferation of astrocytes in the corpus callosum of the rat. J Comp Neurol 137:197–226
Mori S, Leblond CP (1970) Electron microscopic identification of three classes of oligodendrocytes and a preliminary study of their proliferative activity in the corpus callosum of young rats. J Comp Neurol 139:1–30
Noble M (1991) Points of controversy in the O-2A lineage: clocks and type-2 astrocytes. Glia 4:157–164
Noble M, Murray K (1984) Purified astrocytes promote the in vitro division of a bipotential glial progenitor cell. EMBO J 3:2243–2247
Ovadia H, Lubetzki-Korn I, Brenner T, Abramsky O, Fridman R, Vlodavsky I (1984) Adult rat oligodendrocytes grown in vitro upon an extracellular matrix have the ability to proliferate. Brain Res 322:93–100
Prayoonwiwat N, Rodriguez M (1993) The potential for oligodendrocyte proliferation during demyelinating disease. J Neuropathol Exp Neurol 52:55–63
Quinn B, Toga AW (1993) Human astroglial architectonics with a sensitive GFAP immunoimpregnation (abstract). J Histochem Cytochem 41:1129
Raff MC, Mirsky R, Fields KL, Lisak RP, Dorfman SH, Silberberg DE, Gregson NA, Leibowitz S, Kennedy MC (1978) Galactocerebroside is a specific cell-surface antigenic marker for oligodendrocytes in culture. Nature 274:813–816
Raff MC, Abney ER, Cohen J, Lindsay R, Noble M (1983) Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J Neurosci 3:1289–1300
Reyners H, Gianfelici de Reyners E, Maisin JR (1982) The beta astrocyte: a newly recognized radiosensitive glial cell type in the cerebral cortex. J Neurocytol 11:967–983
Reyners H, Gianfelici de Reyners E, Regniers L, Maisin JR (1986) A glial progenitor cell in the cerebral cortex of the adult rat J Neurocytol 15:53–61
Reynolds R, Wilkin GP (1991) Oligodendroglial progenitor cells but not oligodendroglia divide during normal development of the rat cerebellum. J Neurocytol 20:216–224
Richardson KC, Jarett L, Finke EH (1960) Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol 35:313–323
Roussel G, Sensenbrenner M, Labourdette G, Wittendorp-Rechenmann E, Pettmann B, Nussbaum JL (1983) An immunohistochemical study of two myelin-specific proteins in enriched oligodendroglial cell cultures combined with an autoradiographic investigation using 3H-thymidine. Dev Brain Res 8:193–204
Rozeik C, Keyserlingk D von (1987) The sequence of myelination in the brainstem of the rat monitored by myelin basic protein immunohistochemistry. Dev Brain Res 35:183–190
Schultze B, Korr H (1981) Cell kinetic studies of different cell types in the developing and adult brain of the rat and the mouse: a review. Cell Tissue Kinet 14:309–325
Shehab SAS, Cronly-Dillon JR, Nona SN, Stafford CA (1990) Preferential histochemical staining of protoplasmic and fibrous astrocytes in rat CNS with GFAP antibodies using different fixatives. Brain Res 518:347–352
Shimada M, Akagi N, Goto, H, Watanabe, H, Nakanishi, M, Hirose, Y, Watanabe, M (1992) Microvessel and astroglial cell densities in the mouse hippocampus. J Anat 180:89–95
Skoff R, Knapp PE (1991) Division of astroblasts and oligodendroblasts in postnatal rodent brain: evidence for separate astrocyte and oligodendrocyte lineages. Glia 4:165–174
Sternberger LA (1979) Immunocytochemistry. Wiley, New York
Sternberger NH, Del Cerro C, Kies MW, Herndon RM (1985) Immunocytochemistry of myelin basic proteins in adult rat oligodendroglia. J Neuroimmunol 7:355–363
Sturrock RR, McRae DA (1980) Mitotic division of oligodendrocytes which have began myelination. J Anat 131:577–582
Suzumura A, Silberberg DH (1985) Expression of H-2 antigen on oligodendrocytes is induced by soluble factors from concanavalin A activated T cells. Brain Res 336:171–175
Warrington AE, Pfeiffer SE (1992) Proliferation and differentiation of O4+ oligodendrocytes in postnatal rat cerebellum: analysis in unfixed tissue slices using anti-glycolipid antibodies. J Neurosci Res 33:338–353
Wood PM, Bunge RP (1986) Evidence that axons are mitogenic for oligodendrocytes isolated from adult animals. Nature 320:756–758
Wood PM, Bunge RP (1991) The origin of remyelinating cells in the adult central nervous system: the role of the mature oligodendrocyte. Glia 4:225–232
Wood PM, Williams AK (1984) Oligodendrocyte proliferation and CNS myelination in cultures containing dissociated embryonic neuroglia and dorsal root ganglion neurons. Dev Brain Res 12:225–241
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Korr, H., Horsmann, C., Schürmann, M. et al. Problems encountered when immunocytochemistry is used for quantitative glial cell identification in autoradiographic studies of cell proliferation in the brain of the unlesioned adult mouse. Cell Tissue Res 278, 85–95 (1994). https://doi.org/10.1007/BF00305780
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00305780