Summary
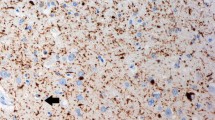
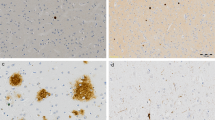
A prospective longitudinal study was undertaken in a geriatric hospital on women over 75 years of age, clinically diagnosed as either intellectually normal or having senile dementia of the Alzheimer type (SDAT) of varying degrees of severity. Mental impairment was assessed prospectively. Fifteen brains from this population were studied to evaluate quantitatively the distribution of senile plaques (SP) in relation to cortical lamination. SP density in four neocortical areas (first temporal gyrus; supramarginal gyrus calcarine area; precentral gyrus) was significantly correlated with the degree of mental impairment. SP distribution in the cortical layers was evaluated by an indirect method and appeared to be fairly constant from one case to another. Significantly higher SP densities were observed in layers II and III of the temporal and occipital samples, while minimal values were noted in layer I. Lower densities of SP were found in layers V and IV of the occipital and temporal lobes. These data suggest a selective vulnerability of some areas of cortical projections in SDAT.
Similar content being viewed by others
References
Arendt T, Bigl V, Tennstedt A, Arendt A (1985) Neuronal loss in different parts of the nucleus basalis is related to neuritic plaque formation in cortical target areas in Alzheimer disease. Neuroscience 14:1–14
Berger B, Escourolle R, Moyne MA (1976) Axones catécholaminergiques du cortex cérébral humain. Observation en histofluorescence de biopsies cérébrales dans deux cas de maladie d'Alzheimer. Rev Neurol (Paris) 132:183–194
Blessed G, Tomlinson BE, Roth M (1968) The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry 114:797–811
Brun A, Englund E (1981) Regional pattern of degeneration in Alzheimer's disease neuronal loss and histopathological grading. Histopathology 5:549–564
Dayan AD (1970) Quantitative histological studies on aged human brain. I. Senile plaques and neurofibrillary tangles in “normal” patients. Acta Neuropathol (Berl) 16:85–94
Duyckaerts C, Hauw JJ, Piette F, Rainsard C, Poulain V, Berthaux P, Escourolle R (1985) Cortical atrophy is mainly due to a decrease in cortical length. Acta Neuropathol (Berl) 66:72–74
Foncin JF, Lebeau J (1965) Ultrastructure des plaques séniles. Rev Neurol (Paris) 112:61–62
Gibson PH (1983) Form and distribution of senile plaques seen in silver impregnated sections in the brains of intellectually normal elderly people and people with Alzheimer type dementia. Neuropathol App Neurobiol 9:379–389
Gilbert CD (1985) Horizontal integration in the neocortex. Trends in Neurosciences 8:160–165
Gonatas NK, Anderson W, Evangelista I (1967) The contribution of altered synapses in the senile plaque: an electron microscopic study in Alzheimer's dementia. J Neuropathol Exp Neurol 26:25–39
Hauw JJ, Vignolo P, Duyckaerts C, Beck H, Forette F, Henry JF, Laurent M, Piette F, Sachet A, Berthaux P (1986) Etude neuropathologique de 12 centenaires: la fréquence de la démence sénile de type Alzheimer ne s'élève pas chez les personnes très âgées. Rev Neurol (Paris) (in press)
Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL (1984) Alzheimer's disease: cell-specific pathology isolates hippocampal formation. Science 225:1168–1170
Kirkpatrick JB (1985) Non-random distribution of senile plaques in cerebral cortex. Abstract of the 61 st Annual Meeting of the American Association of Neuropathologists. J Neuropathol Exp Neurol 44:72
Kitt CA, Price DL, Struble RG, Cork LC, Wainer BH, Becher MW, Mobley WC (1984) Evidence for cholinergic neurites in senile plaques. Science 226:1443–1445
Morrison JH, Magistretti PJ (1983) Monoamines and peptides in cerebral cortex. Contrasting principles of cortical organization. Trends in Neurosciences 6:146–150
Morrison JH, Rogers J, Scherr S, Benoit R, Bloom FE (1985) Somatostatin immunoreactivity in neuritic plaques of Alzheimer's patients. Nature 314:90–92
Pearson RCA, Esiri MM, Hiorns RW, Wilcock GK, Powell TPS (1985) Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc Natl Acad Sci USA 82:4531–4534
Perry EK (1979) Correlations between psychiatric, neuropathological and biochemical findings in Alzheimer's disease. In: Glen AIM, Whalley LJ (eds) Alzheimer's disease. Early recognition of potentially reversible deficits. Churchill Livingstone, Edinburgh, pp 27–35
Perry EK, Tomlinson BE, Blessed G, Bergmann K, Gibson PH, Perry RH (1978) Correlation of cholinergic abnormalities with senile plaques and mental test score in senile dementia. Br Med J 2:1457–1459
Piette F, Duyckaerts C, Laforestrie R, Poulain V, Rainsard C, Sonnette C, Hauw JJ, Berthaux P (1985) Description of the Charles Foix longitudinal study of senile dementia. XIIIth International Congress of Gerontology, New York, July 12–17
Probst A, Basler V, Bron B, Ulrich J (1983) Neuritic plaques in senile dementia of the Alzheimer type: a Golgi analysis in the hippocampal region. Brain Res 268:249–254
Rudelli RD, Ambler MW, Wisniewski HM (1984) Morphology and distribution of Alzheimer neuritic (senile) and amyloid plaques in striatum and diencephalon. Acta Neuropathol (Berl) 64:273–281
Struble RG, Cork LC, Whitehouse PJ, Price DL (1982) Cholinergic innervation in neuritic plaques. Science 316:413–415
Struble RG, Powers RE, Casanova MF, Kitt CA, O'Connor DT, Price DL (1985) Multiple transmitter — specific markers in senile plaques in Alzheimer's disease. J Neuropathol Exp Neurol 44:325
Terry RD, Gonates JK, Weiss M (1964) Ultrastructural studies in Alzheimer presenile dementia. Am J Pathol 44:269–297
Toga M, Tripier-Gouzon MF, Gastaut JL (1970) Maladie d'Alzheimer-Ultrastructure des lésions corticales. In: Vleme Congrès International de Neuropathologie. Masson, Paris, pp 1060–1061
Tomlinson BE, Blessed G, Roth M (1968) Observations on the brains of non-demented old people. J Neurol Sci 7:331–336
Tomlinson BE, Blessed G, Roth M (1970) Observations on the brains of demented old people. J Neurol Sci 11:205–242
Tomlinson BE, Irving D, Blessed G (1981) Cell loss in the locus coeruleus in senile dementia of Alzheimer. J Neurol Sci 49:419–428
Tripier MF, Gambarelli D, Hassoun J, Bérard M, Toga M (1974) La plaque sénile. Ann Anat Pathol 19:6–28
Braunmühl A von (1957) Alterserkrankungen des Zentralnervensystems. Senile Involution. Senile Demenz. Alzheimersche Krankheit. In: Scholz W (ed) Handbuch der speziellen pathologischen Anatomie und Histologie, vol XIII/1A. Springer, Berlin Heidelberg New York, pp 337–539
Economo C von (1927) L'architecture cellulaire normale de l'écorce cérébrale. Translated by L. Van Bogaert. Masson, Paris
Wilcock GK, Esiri MM (1982) Plaques, tangles and dementia. A quantitative study. J Neurol Sci 56:343–356
Wilcock GK, Esiri MM, Bowen DM, Smith CCT (1982) Alzheimer's disease. Correlation of cortical choline acetyltransferase activity with the severity of dementia and histological abnormalities. J Neurol Sci 57:407–417
Wildi E, Dago-Akribi A (1968) Altérations cérébrales chez l'homme âgé. Bull Swiss Acad Med Sci 24:107–132
Wisniewski HM, Terry RD (1973) Reexamination of the pathogenesis of the senile plaque. In: Zimmermann HM (ed) Progress in neuropathology, vol 2. Grune and Stratton, New York, pp 1–26
Author information
Authors and Affiliations
Additional information
Partly supported by INSERM (PRC Santé Mentale et Cerveau no. 133015) and FRMF
Rights and permissions
About this article
Cite this article
Duyckaerts, C., Hauw, J.J., Bastenaire, F. et al. Laminar distribution of neocortical senile plaques in senile dementia of the alzheimer type. Acta Neuropathol 70, 249–256 (1986). https://doi.org/10.1007/BF00686079
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00686079