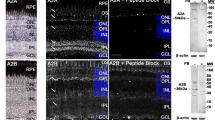
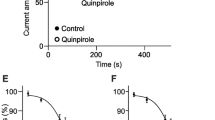
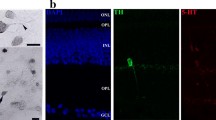
Summary
-
1.
The uptake of [3H] adenosine into specific populations of cells in the inner retina has been demonstrated. In mammalian retina, the exogenous adenosine that is transported into cells is phosphorylated, thereby maintaining a gradient for transport of the purine into the cell.
-
2.
Endogenous stores of adenosine have been demonstrated by localization of cells that are labeled for adenosine-like immunoreactivity. In the rabbit retina, certain of these cells, the displaced cholinergic, GABAergic amacrine cells, are also labeled for adenosine.
-
3.
Purines are tonically released from dark-adapted rabbit retinas and cultured embryonic chick retinal neurons. Release is significantly increased with K+ and neurotransmitters. The evoked release consists of adenosine, ATP, and purine metabolites, and while a portion of this release is Ca2+ dependent, one other component may occur via the bidirectional purine nucleoside transporter.
-
4.
Differential distributions of certain enzymes involved in purine metabolism have also been localized to the inner retina.
-
5.
Heterogeneous distributions of the two subtypes of adenosine receptors, A1 and A2, have been demonstrated in the mammalian retina. Coupling of receptors to adenylate cyclase has also been demonstrated.
-
6.
Adenosine A1 receptor agonists significantly inhibit the K+-stimulated release of [3H]-acetylcholine from the rabbit retina, suggesting that endogenous adenosine may modulate the light-evoked or tonic release of ACh.
Similar content being viewed by others
References
Barnes, S., and Hille, B. (1989). Ionic channels of the inner segment of tiger salamander cone photoreceptors.J. Gen. Physiol. 94719–743.
Bender, A. S., and Hertz, L. (1986). Similarities of adenosine uptake systems in astrocytes and neurons in primary cultures.Neurochem. Res. 111507–1524.
Bender, A. S., Wu, P. H., and Phillis, J. W. (1981). The rapid uptake and release of [3H]adenosine by rat cerebral cortical synaptosomes.J. Neurochem. 36651–660.
Blazynski, C. (1987). Adenosine A1 receptor-mediated inhibition of adenylate cyclase in rabbit retina.J. Neurosci. 72522–2528.
Blazynski, C. (1989a). Identification and localization of adenosine receptors and adenosinergic neurons in mammalian retinas.J. Neurochem. 52S148-S148 (abstr.).
Blazynski, C. (1989b). Displaced cholinergic, GABAergic amacrine cells in the rabbit retina also contain adenosine.Vis. Neurosci. 3425–431.
Blazynski, C. (1990). Discrete distributions of adenosine receptors in mammalian retina.J. Neurochem. 54648–655.
Blazynski, C., Kinscherf, D. A., Geary, K. M., and Ferrendelli, J. A. (1986). Adenosine-mediated regulation of cyclic AMP levels in isolated incubated retinas.Brain Res. 366224–229.
Blazynski, C., Cohen, A. I., Früh, B., and Niemeyer, G. (1989a). Adenosine: autoradiographic localisation and electrophysiological effects in the cat retina.Invest. Ophthalmol. Vis. Sci. 302533–2536.
Blazynski, C., Mosinger, J. L., and Cohen, A. I. (1989b). Comparison of adenosine uptake and endogenous adenosine-containing cells in mammalian retina.Vis. Neurosci. 2109–116.
Braas, K. M., Newby, A. C., Wilson, V. S., and Snyder, S. H. (1986). Adenosine-containing neurons in the brain localized by immunocytochemistry.J. Neurosci. 61952–1961.
Braas, K. M., Zarbin, M. A., and Snyder, S. H. (1987). Endogenous adenosine and adenosine receptors localized to ganglion cells of the retina.Proc. Natl. Acad. Sci. USA 843906–3910.
Campochiaro, P., Ferkany, J. W., and Coyle, J. T. (1985). Excitatory amino acid analogs evoke release of endogenous amino acids and acetyl choline from chick retinain vitro.Vis. Res. 251375–1386.
Cooper, D. M. F., Young, S. M. H., Perez-Reyes, E., Owens, J. R., Fossom, L. H., and Gill, D. L. (1985). Properties required of a functional Ni, the GTP regulatory complex that mediates the inhibitory actions of neurotransmitters on adenylate cyclase.Adv. Cyclic Nucleotide Prot. Phos. Res. 975–86.
Dawis, S., and Niemeyer, G. (1987). Theophylline abolishes the light peak in perfused cat eyes.Invest. Ophthalmol. Vis. Sci. 28700–706.
Deckert, J., Bisserbe, J.-C., Klein, E., and Marangos, P. J. (1988). Adenosine uptake sites in brain: Regional distribution of putative subtypes in relationship to adenosine A1 receptors.J. Neurosci. 82238–2249.
de Mello, M. C. F., Ventura, A. L. M., Paes de Carvelho, R., Klein, W. L., and de Mello, F. G. (1982). Regulation of dopamine- and adenosine-dependent adenylate cyclase systems of chicken embryo retina cells in culture.Proc. Natl. Acad. Sci. USA 795708–5712.
Donner, K., and Hemila, S. (1985). Rhodopsin phosphorylation inhibited by adenosine in frog rods: Lack of effects on excitation.Comp. Biochem. Physiol. 81A431–439.
Dragunow, M., and Faull, R. L. M. (1988). Neuroprotective effects of adenosine.Trends Pharmacol. Sci. 9193–194.
Dunwiddie, T. V. (1985). The physiological role of adenosine in the central nervous system. InInternational Review of Neurobiology (J. R. Symthies, and R. J. Bradley, Eds.), Academic Press, New York, pp. 63–139.
Dunwiddie, T. V., and Fredholm, B. B. (1984). Adenosine receptors mediating inhibitory electrophysiological responses in rat hippocampus are different from receptors mediating cyclic AMP accumulation.Naunyn-Schmiedeberg Arch. Pharmacol. 326294–301.
Dunwiddie, T. V., Hoffer, B. J. and Fredholm, B. B. (1981). Alkylxanthines elevate hippocampal excitability. Evidence for a role of endogenous adenosine.Naunyn-Schmiedeberg Arch. Pharmacol. 316326–330.
Ehinger, B., and Dowling, J. (1987). Retinal neurocircuitry and transmission. InHandbook of Chemical Neuroanatomy, Vol. 5. Integrated Systems of the CNS Part I (A. Bjorklund, T. Hokfelt, and L. W. Swanson, Eds.), Elsevier, Amsterdam, pp. 389–446.
Ehinger, B., and Perez, M. T. R. (1984). Autoradiography of nucleoside uptake into the retina.Neurochem. Int. 6369–381.
Fastbom, J., Pazos, A., and Palacios, J. M. (1987a). The distribution of adenosine A1 receptors and 5′-nucleotidase in the rat brain of some commonly used experimental animals.Neuroscience 22813–826.
Fastbom, J., Pazos, A., Probst, A., and Palacios, J. M. (1987b). Adenosine A1 receptors in the human brain: A quantitative autoradiographic study.Neuroscience 22827–839.
Friedman, Z., Hackett, S. F., Linden, J. and Campochiaro, P. A. (1989). Human retinal pigment epithelial cells in culture possess A2-adenosine receptors.Brain Res. 49229–35.
Geiger, J. D. (1986). Localization of [3H]cyclohexyladenosine and [3H]nitrobenzylthioinosine binding sites in rat striatum and superior colliculus.Brain Res. 363404–408.
Geiger, J. D., and Nagy, J. I. (1984). Heterogeneous distribution of adenosine transport sites labelled by [3H]nitrobenzylthioinosine in rat brain: An autoradiographic and membrane binding study.Brain Res. Bull. 13657–666.
Geiger, J. D., Johnston, M. E., and Yago, V. (1988). Pharmacological characterization of rapidly accumulated adenosine by dissociated brain cells from adult rat.J. Neurochem. 51283–291.
Goodman, R. R., Cooper, M. J., Gavish, M., and Snyder, S. H. (1982). Guanine nucleotide and cation regulation of the binding of [3H]cyclohexyladenosine and [3H]diethylphenylxanthine to adenosine A1-receptors in brain membranes.Mol. Pharmacol. 21329–335.
Goodmam, R. R., Kuhar, M. J., Hester, L., and Snyder, S. H. (1983). Adenosine receptors: Autoradiographic evidence for their location on axon terminals of excitatory neurons.Science 220967–969.
Henderson, J. F. (1979). Regulation of adenosine metabolism. InPhysiological and Regulatory Functions of Adenosine and Adenine Nucleotides (H. P. Baer, and G. I. Drummond, Eds.), Raven Press, New York, pp. 315–322.
Hollins, C., and Stone, T. W. (1980). Characteristics of the release of adenosine from slices of rat cerebral cortex.J. Physiol. 30373–82.
Jarvis, S. M. (1988). Adenosine transporters. InReceptor Biochemistry and Methodology: Adenosine Receptors (D. M. F. Cooper, and C. Londos, Eds.), Alan R. Liss, New York, pp. 113–123.
Jarvis, M. F., Jackson, R. H., and Williams, M. (1989). Autoradiographic characterization of high-affinity adenosine A2 receptors in the rat brain.Brain Res. 484111–118.
Jonzon, B., and Fredholm, B. B. (1985). Release of purines, noradrenaline and GABA from rat hippocampal slices by field stimulation.J. Neurochem. 44217–224.
Kreutzberg, G. W., and Hussain, S. T. (1984). Cytochemical localization of 5′-nucleotidase activity in retinal photoreceptor cells.Neuroscience 11 857–866.
Kreutzberg, G. W., Barron, K. D., and Schubert, P. (1978). Cytochemical localization of 5′-nucleotidase in glial plasma membranes.Brain Res. 158247–257.
LeHir, M., and Dubach, U. C. (1984). Sodium gradient-energized concentrative transport of adenosine in renal brush border vesicles.Pflugers Arch. 40158–63.
LeHir, M., and Dubach, U. C. (1985a). Uphill transport of pyrimidine nucleotides in renal brush border vesicles.Pflugers Arch. 404238–243.
LeHir, M., and Dubach, U. C. (1985b). Concentrative transport of purine nucleosides in brush border vesicles of the rat kidney.Eur. J. Clin. Invest. 15121–127.
Lohse, M. J., Lenschow, V., and Schwabe, U. (1984). Two affinity states of Ri adenosine receptors in brain membranes.Mol. Pharmacol. 261–9.
Londos, C., Wolff, J., and Cooper, D. M. F. (1981). Adenosine as a regulator of adenylate cyclase. InPurine Receptors, Receptors and Recognition, Series B, Vol. 12 (G. Burnstock, Ed.), Chapman and Hall, London, pp. 289–323.
MacDonald, W. F., and White, T. D. (1985). Nature of extrasynaptosomal accumulation of endogenous adenosine evoked by K+ and verayridine.J. Neurochem. 45791–797.
Magistretti, P. J., Hof, P. R., and Martin, J.-L. (1986). Adenosine stimulates glycogenolysis in mouse cerebral cortex: A possible coupling mechanism between neuronal activity and energy metabolism.J. Neurosci. 62558–2562.
Masland, R. H., Mills, J. W., and Hayden, S. A. (1984). Acetylcholine-synthesizing amacrine cells: Identification and selective staining by using radioautography and fluorescent markers.Proc. R. Soc. Lond. 22379–100.
Massey, S. C., and Redburn, D. A. (1987). Transmitter circuits in the vertebrate retina.Prog. Neurobiol. 2855–96.
Michaelis, M. L., Johe, K. K., Moghadam, B., and Adams, R. N. (1988). Studies on the ionic mechanism for the neuromodulatory actions of adenosine in the brain.Brain Res. 473249–260.
Motley, S. J., and Collins, G. G. S. (1983). Endogenous adenosine inhibits excitatory transmission in the rat olfactory cortex slice.Neuropharmacology 221081–1086.
Nagy, J. I., LaBella, L. A., Buss, M., and Daddona, P. E. (1984). Immunohistochemistry of adenosine deaminase: Implications for adenosine neurotransmission.Science 224166–168.
Nagy, J. I., Geiger, J. D., and Daddona, P. E. (1985). Adenosine uptake sites in rat brain: Identification using [3H]nitrobenzylthioinosine and colocalization with adenosine deaminase.Neurosci. Lett. 5547–53.
Newby, A. C., and Sala, G. B. (1982). A new procedure for haptenizing adenosing leading to a more specific radioimmunoassay method.Biochem. J. 208603–610.
Niemeyer, G., and Früh, B. (1989). Adenosine and cyclohexyladenosine inhibit the cat's optic nerve action potential.Experientia 45:A18 (abstr.).
Osborne, N. N. (1989). [3H]Glycogen hydrolysis elicited by adenosine in rabbit retina: Involvement of A2-receptors.Neurochem. Int. 14419–422.
Paes de Carvalho, R. (1990). Development of A1 adenosine receptors in the chick embryo retina.J. Neurosci. Res. 25236–242.
Paes de Carvalho, R., and de Mello, F. G. (1982). Adenosine-elicited accumulation of adenosine 3′,5′-cyclic monophosphate in the chick embryo retina.J. Neurochem. 38493–500.
Paes de Carvalho, R., and de Mello, F. G. (1985). Expression of A1 adenosine receptors modulating dopamine-dependent cyclic AMP accumulation in the chick embryo retina.J. Neurochem. 44845–851.
Paes de Carvalho, R., Braas, K. M., Snyder, S. H., and Adler, R. (1989). Adenosine uptake and release in purified cultures of chick retinal neurons and photoreceptors.J. Neurochem. 52s157-s157 (abstr.).
Paes de Carvalho, R., Braas, K. M., Snyder, S. H., and Adler, R. (1990). Analysis of adenosine immunoreactivity, uptake and release in purified cultures of developing chick embryo retinal neurons and photoreceptors.J. Neurochem. 551603–1611.
Palczewski, K., McDowell, J. H., and Hargrave, P. H. (1988). Purification and characterization of rhodopsin kinase.J. Biol. Chem. 26314067–14073.
Perez, M. T. R., and Bruun, A. (1987). Colocalization of [3H]-adenosine accumulation and GABA immunoreactivity in the chicken and rabbit retinas.Histochemistry 87413–417.
Perez, M. T. R., and Ehinger, B. (1986). Adenosine uptake and release in the rabbit retina. InRetinal Signal Systems, Degenerations and Transplants (E. Agardh, and B. Ehinger, Eds.), Elsevier Science, Amsterdam, pp. 163–172.
Perez, M. T. R., and Ehinger, B. (1989a). Multiple neurotransmitter systems influence the release of adenosine derivatives from the rabbit retina.Neurochem. Int. 15411–420.
Perez, M. T. R., and Ehinger, B. (1989b). Adenosine inhibits evoked acetylcholine release from the rabbit retina.J. Neurochem. 52:S157 (abstr.).
Perez, M. T. R., Ehinger, B. E., Linstrom, K., and Fredholm, B. B. (1986). Release of endogenous and radioactive purines from the rabbit retina.Brain Res. 398106–112.
Perez, M. T. R., Arner, K., and Ehinger, B. (1988). Stimulation-evoked release of purines from the rabbit retina.Neurochem. Int. 13307–318.
Phillis, J. W., and Wu, P. H. (1981). The role of adenosine and its nucleotides in central synaptic transmission.Prog. Neurobiol. 16187–239.
Schorderet, M. (1989). Receptors coupled to adenylate cyclase in isolated rabbit retina.Neurochem. Int. 14387–395.
Schubert, P. (1988). Physiological modulation by adenosine: selective blockade of A1 receptors with DPCPX enhances stimulus train-evoked neuronal Ca influx in rat hippocampal slices.Brain Res. 458162–165.
Scott, T. G. (1967). The distribution of 5′-nucleotidase in the brain of the mouse.J. Comp. Neurol. 12997–113.
Senba, E., Daddona, P. E., and Nagy, J. I. (1986). Immunohistochemical localization of adenosine deaminase in the retina of the rat.Brain Res. Bull. 17209–217.
Tapia, P., and Arias, C. (1982). Selective stimulation of neurotransmitter release from chick retina by kainic acid and glutamic acid.J. Neurochem. 391169–1178.
Tauchi, M., and Masland, R. H. (1984). The shape and arrangement of the cholinergic neurons in the rabbit retina.Proc. R. Soc. Lond. 223101–119.
van Calker, D., Muller, M., and Hamprecht, B. (1979). Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells.J. Neurochem. 33999–1005.
Watt, C. B., Su, Y. Y. T., and Lam, D. M. K. (1985). Enkaphalins in the vertebrate retina. InProgress in Retinal Research, Vol. 4 (N. N. Osborne, and G. J. Chader, Eds.), Pergamon Press, Oxford, pp. 221–242.
Williams, M. (1987). Purine receptors in mammalian tissues: Pharmacology and functional significance.Annu. Rev. Pharmacol. Toxicol. 27315–345.
Woods, C., and Bazynski, C. (1991). Characterization of adenosine A1 receptor binding sites in bovine retinal membranes.Exp. Eye Res. (in press).
Wu, P. H., and Phillis, J. W. (1984). Uptake by central nervous tissues as a mechanism for the regulation of extracellular adenosine concentrations.Neurochem. Int. 6613–632.
Wu, P. H., Moron, M., and Barraco, R. (1984). Organic calcium channel blockers enhance [3H]purine release from rat brain cortical synaptosomes.Neurochem. Res. 91019–1031.
Yeung, S. M., and Green, R. D. (1983). Agonist and antagonist affinities for inhibitory adenosine receptors are reciprocally affected by 5′-guanylylimidodiphosphate or N-ethylmaleimide.J. Biol. Chem. 2582334–2339.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Blazynski, C., Perez, MT.R. Adenosine in vertebrate retina: Localization, receptor characterization, and function. Cell Mol Neurobiol 11, 463–484 (1991). https://doi.org/10.1007/BF00734810
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00734810