Summary
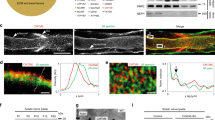
Myelinating Schwann cells polarize their surface membrane into several ultrastructurally and biochemically distinct domains that constitute the myelin internode. Formation of these membrane domains depends on contact with appropriate axons and requires microtubule-based transport systems for site-specific targeting of membrane components. Because little is known about microtubules in myelinating Schwann cells, this study used confocal microscopy and the microtubule hook-labelling method to characterize microtubule distribution, the location of microtubule nucleation sites, and the polarity and composition of Schwann cell microtubules. In myelinating Schwann cells, microtubules were abundant within the Golgi-rich perinuclear cytoplasm; they were not attached to the centrosome. Three-fourths of the microtubules in the cytoplasmic channels located along the outer perimeter of the myelin internode had their (+) ends oriented away from the perinuclear region, whereas the remaining 25% had the opposite polarity. Depolymerization/repolymerization experiments detected microtubule nucleating sites in perinuclear cytoplasm but not along the myelin internode. Taken together, these results indicate that microtubule-mediated transport of myelin components along the internode could utilize both (+)- and (-)-end motors. Specialized microtubule tracks that target myelin proteins to specific sites were not identified on the basis of tubulin polarity or posttranslational modifications.
Similar content being viewed by others
References
Achler, C., Filmer, D., Merte, C. &Drenckhahn, D. (1989) Role of microtubules in polarized delivery of apical membrane proteins to the brush border of the intestinal epithelium.Journal of Cell Biology 109, 179–89.
Avila, J. (1991) Microtubule functions.Life Sciences 50, 327–34.
Baas, P. W., Deitch, J. S., Black, M. M. &Banker, G. A. (1988) Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite.Proceedings of the National Academy of Sciences (USA) 85, 8335–9.
Baas, P. W. &Ahmad, F. J. (1992) The plus ends of stable microtubules are the exclusive nucleating structures for microtubules in the axon.Journal of Cell Biology 116, 1231–41.
Baas, P. W. &Black, M. M. (1990) Individual microtubules in the axon consist of domains that differ in both composition and stability.Journal of Cell Biology 111, 495–509.
Bacallao, R., Antony, C., Dotti, C., Karsenti, E., Stelzer, E. H. K. &Simons, K. (1989) The subcellular organization of Madin-Darby canine kidney cells during the formation of a polarized epithelium.Journal of Cell Biology 109, 2817–32.
Burton, P. R. (1988) Dendrites of mitral cell neurons contain microtubules of opposite polarity.Brain Research 473, 107–15.
Cambray-Deakin, M. &Burgoyne, R. D. (1987) Acetylated and detyrosinated alpha-tubulins are co-localized in stable microtubules in rat meningeal fibroblasts.Cell Motility and the Cytoskeleton 8, 284–91.
Colman, D. R., Kreibich, G., Frey, A. B. &Sabatini, D. D. (1982) Synthesis and incorporation of myelin polypeptide into CNS myelin.Journal of Cell Biology 95, 598–608.
Davis, L., Burger, B., Banker, G. A. &Steward, O. (1990) Dendritic transport: quantitative analysis of the time course of somatodendritic transport of recently synthesized RNA.Journal of Neuroscience 10, 3056–68.
Doxsey, S. J. &Kirschner, M. (1993) Pericentrin, a conserved protein of the centrosome. InCytoskeletal and Structural Proteins (edited byVale, R. &Kreis, T.) in press.
Dustin, P. (1984)Microtubules, 2nd ed. Berlin: Springer-Verlag.
Gilbert, T., Le Bivic, A., Quaroni, A. &Rodriguez-Boulan, E. (1991) Microtubular organization and its involvement in the biogenetic pathways of plasma membrane proteins in Caco-2 intestinal epithelial cells.Journal of Cell Biology 113, 275–88.
Gould, R. M. &Mattingly, G. (1990) Regional localization of RNA and protein metabolism in Schwarm cellsin vivo.Journal of Neurocytology 19, 285–301.
Griffiths, I. R., Mitchell, L. S., Mcphilemy, K., Morri-Son, S., Kyriakides, E. &Barrie, J. A. (1989) Expression of myelin protein genes in Schwann cells.Journal of Neurocytology 18, 345–52.
Heidemann, S. R. &Mcintosh, J. R. (1980) Visualization of the structural polarity of microtubules.Nature 286, 517–19.
Heidemann, S. R., Landers, J. M. &Hamborg, M. A. (1981) Polarity orientation of axonal microtubules.Journal of Cell Biology 91, 661–5.
Heidemann, S. R. (1991) Microtubule polarity determination based on formation of protofilament hooks. InMethods in Enzymology, Vol. 196 (edited byVallee, R. B.) pp. 469–77. New York: Academic Press.
Horio, T., Uzawa, S., Jung, M. K., Oakley, B. R., Tanaka, K. &Yanagida, M. (1991) The fission yeast gamma-tubulin is essential for mitosis and is localized at microtubule organizing centers.Journal of Cell Science 99, 693–709.
Hugon, J. S., Bennett, G., Pothier, P. &Ngoma, Z. (1987) Loss of microtubules and alteration of glycoprotein migration in organ cultures of mouse intestine exposed to nocodazole or colchicine.Cell and Tissue Research 248, 653–62.
Joshi, H. C., Palacios, M. J., Mcnamara, L. &Cleveland, D. W. (1992) Gamma-tubulin is a centrosomal protein required for cell cycle-dependent microtubule nucleation.Nature 356, 80–3.
Kreis, T. E. (1990) Role of microtubules in the organisation of the Golgi apparatus.Cell Motility and the Cytoskeleton 15, 67–70.
Martini, R. &Schachner, M. (1986) Immunoelcctron microscopic localization of neural cell adhesion molecules (L1, N-CAM, and MAG) and their shared carbohydrate epitope and myelin basic protein in developing sciatic nerve.Journal of Cell Biology 103, 2439–48.
Peters, A., Palay, S. L. &Webster, H. Def. (1991).The Fine Structure of the Nervous System: Neurons and their Supporting Cells, 3rd ed. New York: Oxford University Press.
Piperno, G., Ledizet, M. &Chang, X. (1987) Microtubules containing acetylated alpha-tubulin in mammalian cells in culture.Journal of Cell Biology 104, 289–302.
Pryer, N. K., Walker, R. A., Skeen, V. P., Bourns, B. D., Soboeiro, M. F. &Salmon, E. D. (1992) Brain microtubule-associated proteins modulate microtubule dynamic instabilityin vitro: real-time observations using video microscopy.Journal of Cell Science 103, 965–76.
Ramon Y Cajal, S. (1928)Degeneration and regeneration of the nervous system (translated byMay, R. M.) London: Oxford University Press.
Tassin, A.-M., Maro, B. &Bornens, M. (1985) Fate of microtubule-organizing centers during myogenesis in vitro.Journal of Cell Biology 100, 35–46.
Thyberg, J. &Moskalewski, S. (1985) Microtubules and the organization of the Golgi complex.Experimental Cell Research 159, 1–16.
Trapp, B. D. &Quarles, R. H. (1982) Presence of the myelin-associated glycoprotein correlates with alterations in the periodicity of peripheral myelin.Journal of Cell Biology 92, 877–82.
Trapp, B. D., Itoyama, Y., Sternberger, N. H., Quarles, R. H. &Webster, H. Def. (1981) Immunocyto-chemical localization of Po protein in Golgi complex membranes and myelin of developing rat Schwann cells.Journal of Cell Biology 90, 1–6.
Trapp, B. D., Hauer, P. &Lemke, G. (1988) Axonal regulation of myelin protein mRNA levels in actively myelinating Schwann cells.Journal of Neuroscience 8, 3515–21.
Trapp, B. D., Kidd, G. J., Hauer, P. E., Mulrenin, E., Haney, C. &Andrews, S. B. (1994) Polarization of myelinating Schwann cell surface membranes: role of microtubules and the trans-Golgi network. Journal of Neuroscience, in press.
Viancour, T. A. &Forman, D. S. (1987) Polarity orientations of microtubules in squid and lobster axons.Journal of Neurocytology 16, 69–75.
Yisraeli, J. K., Sokol, S. &Melton, D. A. (1990) A two-step model for the localization of maternal mRNA inXenopus oocytes: involvement of microtubules and micro-filaments in the translocation and anchoring of Vg1 mRNA.Development 108, 289–98.
Yu, W., Centonze, V. E., Ahmad, F. J. &Baas, P. W. (1993) Microtubule nucleation and release from the neuronal centrosome.Journal of Cell Biology 122, 349–59.
Zheng, Y., Jung, M. K. &Oakley, B. R. (1991) Gamma-tubulin is present inDrosophila melanogaster andHomo sapiens and is associated with the centrosome.Cell 65, 817–23.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kidd, G.J., Andrews, S.B. & Trapp, B.D. Organization of microtubules in myelinating Schwann cells. J Neurocytol 23, 801–810 (1994). https://doi.org/10.1007/BF01268092
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01268092