Abstract
Aims/hypothesis
Upon stimulation of insulin signalling or contraction-induced AMP-activated protein kinase (AMPK) activation, the glucose transporter GLUT4 and the long-chain fatty acid (LCFA) transporter CD36 similarly translocate from intracellular compartments to the plasma membrane of cardiomyocytes to increase uptake of glucose and LCFA, respectively. This similarity in regulation of GLUT4 traffic and CD36 traffic suggests that the same families of trafficking proteins, including vesicle-associated membrane proteins (VAMPs), are involved in both processes. While several VAMPs have been implicated in GLUT4 traffic, nothing is known about the putative function of VAMPs in CD36 traffic. Therefore, we compared the involvement of the myocardially produced VAMP isoforms in insulin- or contraction-induced GLUT4 and CD36 translocation.
Methods
Five VAMP isoforms were silenced in HL-1 cardiomyocytes. The cells were treated with insulin or the contraction-like AMPK activator oligomycin or were electrically stimulated to contract. Subsequently, GLUT4 and CD36 translocation as well as substrate uptake were measured.
Results
Three VAMPs were demonstrated to be necessary for both GLUT4 and CD36 translocation, either specifically in insulin-treated cells (VAMP2, VAMP5) or in oligomycin/contraction-treated cells (VAMP3). In addition, there are VAMPs specifically involved in either GLUT4 traffic (VAMP7 mediates basal GLUT4 retention) or CD36 traffic (VAMP4 mediates insulin- and oligomycin/contraction-induced CD36 translocation).
Conclusions/interpretation
The involvement of distinct VAMP isoforms in both GLUT4 and CD36 translocation indicates that CD36 translocation, just like GLUT4 translocation, is a vesicle-mediated process dependent on soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex formation. The ability of other VAMPs to discriminate between GLUT4 and CD36 translocation allows the notion that myocardial substrate preference can be modulated by these VAMPs.
Similar content being viewed by others
Introduction
Glucose and long-chain fatty acids (LCFA) are the predominant substrates for energy provision of the contracting heart. The major cardiac substrate transporters in the heart are GLUT4 (glucose) and CD36 (LCFA), both of which cycle between intracellular stores and the plasma membrane (for review see Schwenk et al. [1]). Translocation of GLUT4 and CD36 to the plasma membrane is induced under conditions of elevated insulin levels (e.g. postprandially) and under conditions of increased ATP demand (e.g. upon increased contraction), involving activation of insulin signalling and AMP-activated protein kinase (AMPK), respectively.
In the early insulin-resistant heart there is a permanent relocation of CD36 from endosomes to the plasma membrane while GLUT4 distribution appears not yet affected [2]. The constantly elevated presence of CD36 at the plasma membrane fuels a vicious cycle of increased CD36-mediated LCFA uptake and subsequent accumulation of toxic lipid metabolites (lipotoxicity) [2, 3]. Over time, lipotoxicity will lead to aggravation of insulin resistance, and finally to cardiac dysfunction [4]. In order to explain the selective increase in sarcolemmal content of CD36 in the early insulin-resistant heart, there must be alterations in the trafficking machinery of CD36. Given that sarcolemmal CD36 relocation is an early key event in diabetes-related cardiac dysfunction, it is of pivotal importance to identify the trafficking machinery involved in the subcellular distribution of CD36, especially in relation to that of GLUT4.
The subcellular distribution of GLUT4 and CD36 is regulated by soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptors (SNAREs), the mechanistical core complexes of membrane fusion [5]. Historically, SNAREs have been classified into vesicle-associated SNAREs (v-SNAREs) and target-membrane-associated SNAREs (t-SNAREs). However, more recent classifications reflect the conserved amino-acid residues within the SNARE motifs, which are either a glutamine (Q-SNAREs) or an arginine (R-SNAREs). When the vesicle-associated R-SNAREs interact with a specific subset of Q-SNAREs at the target membrane, a hetero-oligomeric SNARE complex is formed that catalyses the fusion of the vesicle with the target membrane.
While there are several subfamilies of t-SNAREs (syntaxins, SNAPs, Sec proteins), vesicle-associated membrane proteins (VAMPs) represent all members of the v-SNARE subfamily [5]. There are seven identified VAMP isoforms: VAMP1/synaptobrevin1, which is involved in synaptic vesicle fusion [6] and appears to be absent in muscle tissues [7]; VAMP2/synaptobrevin2; VAMP3/cellubrevin; VAMP4; VAMP5/myobrevin, which is predominantly produced in heart and skeletal muscle [8]; VAMP7/TI-VAMP; and VAMP8/endobrevin [5]. Insulin-stimulated translocation of GLUT4 involves VAMP2, which interacts with the t-SNAREs syntaxin4 and SNAP23 at the plasma membrane [9, 10]. Furthermore, VAMP7 has been shown to mediate GLUT4 translocation on hyperosmotic shock [11]. These findings suggest that different VAMP isoforms might be involved in the translocation of GLUT4 upon different stimuli. However, nothing is known about the role of VAMPs in CD36 trafficking.
The purpose of this study was to investigate the involvement of each of the myocardial VAMPs in insulin- and contraction-stimulated GLUT4 and CD36 translocation to the plasma membrane. HL-1 atrial cardiomyocytes were used in which the genes encoding the different VAMP isoforms were transiently knocked down using small interfering RNAs (siRNAs). In these cells, plasmalemmal GLUT4 and CD36, as well as glucose and palmitate uptake, were measured after activation of insulin or contraction signalling. The data presented in this study disclose the involvement of distinct VAMPs in the cellular distribution of GLUT4 and CD36 and their importance for the insulin- and contraction-stimulated translocation of both transporters to the plasma membrane of cardiomyocytes.
Methods
Antibodies, siRNAs and plasmids
Antibodies against VAMP2 and VAMP3 were obtained from Abcam (Cambridge, UK), against VAMP4 from Novus Biologicals (Littleton, CO, USA), against VAMP5 from Synaptic Systems (Göttingen, Germany) and against VAMP7 and GLUT4 from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against the myc epitope, protein kinase B (PKB/AKT), phospho-PKB/AKT(Ser473), phospho-acetyl-coA carboxylase (pACC; Ser79), phospho-AMPKα (pAMPK; Thr172) and cleaved caspase-3 were from Cell Signaling (Danvers, MA, USA), and against mouse CD36 from Chemicon (Billerica, MA, USA). siRNAs against VAMP2/synaptobrevin2 (CCGATCTTTCCCTTATCTTtt), VAMP3/cellubrevin (GCTCATGCTCTTATGTTAGtt), VAMP4 (CGTACGTTTGAGCTTATAAtt), VAMP5/myobrevin (GGGAAGGCTGAATGACTGCtt) and VAMP7/TI-VAMP (GGAAATTCATGTGACTATGtt) were from Ambion (Applied Biosystems, Austin, TX, USA). The plasmid coding for GLUT4myc was kindly provided by J. Eckel (German Diabetes Center, Düsseldorf, Germany).
Cell culture of HL-1 atrial cardiomyocytes
HL-1 cells were kindly provided by W. Claycomb (Louisiana State University, New Orleans, LA, USA), cultured in Claycomb medium (supplemented with 10% FCS, 0.1 mmol/l noradrenaline [norepinephrine], 2 mmol/l l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin) at 37°C and 5% CO2. Cells were split twice a week after reaching confluence.
Electric-field stimulation
HL-1 cells were electrically paced using the C-Pace Stimulator and six-well C-Dish Culture Dish Electrodes from IonOptix (Milton, MA, USA). Prior to electric-field stimulation, cells were incubated in depletion medium (DMEM low glucose, supplemented with 2 mmol/l l-glutamine, 100 μmol/l non-essential amino acids (NEAA), 100 U/ml penicillin and 100 μg/ml streptomycin) for 16 h at 37°C and 5% CO2. Electric-field stimulation was performed for 30 min at 40 V with a frequency of 2 Hz and a pulse duration of 15 ms.
Content of adenosine phosphates
Pelleted HL-1 cells were freeze dried then extracted with 1.3 mol/l perchloric acid and neutralised with 1 mol/l KHCO3. Thereafter, concentrations of ATP, ADP and AMP were determined by high-performance liquid chromatography as described previously by Luiken et al. [12].
Transfection of HL-1 cells
Plates (48 well) were seeded with 80,000 cells per well 24 h before transfection with double-stranded siRNAs. Transfection of siRNA was done with 25 pmol siRNA and 1 μl Lipofectamine-2000 (Invitrogen, San Diego, CA, USA) per well in antibiotic- and noradrenaline-free culture medium. Cotransfection of siRNA with pCMV-GLUT4myc was performed with 20 pmol siRNA, 0.15 μg plasmid DNA and 1 μl Lipofectamine-2000 per well in antibiotic- and noradrenaline-free culture medium. After 6 h, the medium was changed to regular culture medium. Cell lysis or functional assays were performed 48 h after siRNA transfection.
CD36 cell surface staining
Plasmalemmal CD36 was measured 48 h after transfection of siRNA into HL-1 cardiomyocytes. Prior to measuring CD36, cells were incubated in depletion medium for 16 h at 37°C and 5% CO2. Translocation of CD36 was induced by incubation with 200 nmol/l insulin or 1 μmol/l oligomycin, or by electric-field stimulation with 2 Hz pacing for 30 min at 37°C and 5% CO2. Afterwards, cells were fixed with 4% (wt/vol.) formaldehyde twice for 10 min at room temperature and blocked for 1 h with PBS containing 1% (wt/vol.) non-fat dried milk and 0.05% (vol./vol.) Tween 20. Cells were incubated with an anti-CD36 antibody (1:2,000 in blocking buffer) for 30 min at room temperature and subsequently washed three times with blocking buffer. Next, cells were incubated with a horseradish peroxidase (HRP)-linked secondary antibody (1:4,000 in blocking buffer) for 30 min at room temperature. After extensive washing with blocking buffer and PBS, an ortho-phenylenediamine–H2O2 solution was added as a substrate for the bound HRP. The reaction was carried out at room temperature and stopped after 30 min by addition of 1 mol/l H2SO4. Colour development, representative of the amount of CD36 present at the plasma membrane, was quantified by measurement of the absorbance at 490 nm. The background signal of the control (incubation without primary antibody) was subtracted from the raw data.
GLUT4myc cell surface staining
In contrast to CD36, GLUT4 is a transmembrane protein without a large extracellular epitope. Therefore, it cannot be detected by an antibody when integrated with the plasma membrane. This problem can be solved by overproduction of a GLUT4 variant carrying an myc tag on its first extracellular epitope [13]. When properly integrated with the plasma membrane the myc epitope of GLUT4myc is extracellular and can easily be detected. In our study, plasmalemmal GLUT4myc was measured 48 h after cotransfection of the plasmid coding for GLUT4myc and the siRNAs. The staining of plasmalemmal GLUT4myc is done with a primary antibody against myc and an appropriate HRP-linked secondary antibody. Aside from these details, the GLUT4myc surface staining is homologous to the method of CD36 cell-surface staining. The background signal of the controls (1, incubation without primary antibody; 2, untransfected cells) was subtracted from the raw data.
Glucose and palmitate uptake
Substrate uptake into HL-1 cardiomyocytes was measured 48 h after transfection of the HL-1 cardiomyocytes seeded on pre-coated glass slides. Serum-depleted cells were treated with 200 nmol/l insulin, 1 μmol/l oligomycin or electrical pacing (2 Hz) for 30 min at 37°C. Subsequently, palmitate (coupled to BSA in a palmitate/BSA ratio of 1:3) and deoxy-d-glucose were added to final concentrations of 20 and 4 μmol/l, respectively, with tracer amounts of [14C]palmitate and 2-deoxy-d-[3H]glucose. After 10 min, uptake was terminated and unbound substrate removed by washing the cells with ice-cold depletion medium containing 0.2 mmol/l phloretin. After transfer of the glass slides into new culture dishes, cells were lysed by addition of 1 mol/l NaOH. Subsequently, incorporated glucose and palmitate were measured by scintillation counting of 14C and 3H.
Cell lysis and western blotting
Cell lysis and western blotting were performed as described earlier by van Oort et al. [14].
Statistics
All data are presented as means ± SEM. Statistical analysis was performed by using Student’s t test or ANOVA (including Newman–Keuls multiple comparison test) and statistical analysis software Prism 4 (GraphPad Software). A p value of <0.05 was considered statistically significant.
Results
Characterisation of HL-1 cells
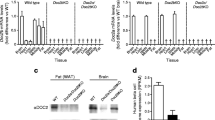
HL-1 cells have previously been shown to produce the cardiac glucose transporters GLUT1 and GLUT4 as well as the fatty acid transporter CD36 [15, 16]. When comparing the production of the different VAMP isoforms in mouse heart, skeletal muscle, brain and HL-1 cells, we observed that VAMP isoforms 2, 3, 4, 5 and 7 are detectable in heart and skeletal muscle as well as in HL-1 cells (Fig. 1a). However, as VAMP8 was only detectable in mouse brain, it was not further investigated.
Characterisation of HL-1 cardiomyocytes. a HL-1 lysates and homogenates from mouse heart (H), skeletal muscle (SkM) and brain (Br) were separated with SDS-PAGE and blotted against VAMP isoforms 2–8. b AMPK activation in HL-1 cells by electric-field stimulation. Serum-depleted cells were electrically paced for 30 min with 40 V, 2 Hz and 15 ms pulses. Lysates were separated by SDS-PAGE and blotted against pAMPK and pACC. Cells treated with 1 μmol/l oligomycin served as a positive control for AMPK and ACC phosphorylation. c Apoptosis in HL-1 cells. Serum-depleted HL-1 cells were stimulated with two concentrations of oligomycin for 30 min. Lysates were separated by SDS-PAGE and blotted for pAMPK and cleaved caspase-3. Staurosporine, 5 μmol/l, was used as a positive control for apoptosis-induced cleaved caspase-3 accumulation. d Selectivity of insulin and oligomycin stimulation on protein phosphorylation. Serum-depleted cells were treated with 200 nmol/l insulin or 1 μmol/l oligomycin for 30 min. Lysates were separated by SDS-PAGE and blotted for pAKT, pAMPK and pACC. Representative western blots are shown
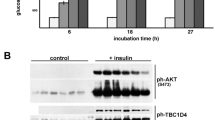
A suitable approach to induce myocellular contractions in vitro is electric-field stimulation, resulting in elevated AMP levels and subsequent activation of AMPK [17]. HL-1 cardiomyocyte cultures were also found to contract upon electric-field stimulation using 40 V and 2 Hz pulses of 15 ms length. Under this condition, the AMP/ATP ratio increased 2.1-fold (Table 1), leading to a subsequent increase in phosphorylation of AMPK (2.8-fold) and ACC (14.0-fold, p < 0.05, n=3; Fig. 1b). Alternatively, ATP depletion, as occurs during contraction, can be achieved by incubation of cardiomyocytes with the F0F1-ATPase inhibitor oligomycin. Already low concentrations of oligomycin (1 μmol/l) resulted in a 2.7-fold increase in the AMP/ATP ratio and a robust increase in phosphorylation of AMPK and ACC (3.6-fold and 11.3-fold, respectively; p < 0.05, n=3; Table 1, Fig. 1b). The use of electric-field stimulation, however, requires a disproportional expenditure compared with the equivalent pharmacological approach with oligomycin. Therefore, the systematic screening for VAMP isoforms involved in AMPK-mediated transporter translocation was performed with oligomycin and the results evaluated afterwards with electric-field-stimulated cells. Oligomycin treatment did not increase apoptosis in HL-1 cells as measured on the level of cleaved caspase-3 (Fig. 1c). In contrast, cleaved caspase-3 accumulated in cells treated with staurosporine, a known initiator of cell death [18]. Activation of insulin signalling was investigated by measuring protein kinase B/AKT Ser473 phosphorylation, which was elevated 3.3-fold by 200 nmol/l insulin (Fig. 1d). Insulin treatment did not interfere with AMPK signalling, nor did oligomycin interfere with insulin signalling (Fig. 1d).
Transfection of HL-1 cells with pCMV-GLUT4myc led to a robust overproduction of GLUT4myc that did not interfere with endogenous CD36 production (Fig. 2a). Each of the stimuli induced the translocation of GLUT4myc (Fig. 2b) and CD36 (Fig. 2c) to the plasma membrane to comparable magnitudes. Subsequent substrate uptake, especially palmitate uptake, has to be corrected for the non-protein-mediated uptake component. This can be done by subtraction of the uptake rate that is not inhibited by phloretin, a general inhibitor of protein-mediated membrane transport [19]. In HL-1 cells, basal glucose uptake was entirely inhibited by phloretin (Fig. 2d), while basal palmitate uptake was inhibited by 52% (Fig. 2e). For comparison, sulfo-N-succinimidyloleate (SSO), a specific inhibitor of CD36, inhibited palmitate uptake by 33% (Fig. 2e), while not affecting glucose uptake (Fig. 2e). Hence, CD36 contributes >60% of the protein-mediated component of basal palmitate uptake. When the cells were treated with insulin, both glucose and palmitate uptake were stimulated (Fig. 2d,e). When SSO was added, insulin-stimulated palmitate uptake, but not glucose uptake, was completely prevented (Fig. 2e). This confirms that CD36 translocation is responsible for the stimulus-induced increase in palmitate uptake. However, total palmitate uptake has to be corrected for the passive diffusion component (phloretin) of palmitate uptake. Therefore, palmitate uptake values in a phloretin-treated group were routinely subtracted from the total palmitate uptake in order to determine facilitated palmitate uptake. Such correction for the passive diffusion component is not necessary in the case of glucose uptake, which is almost entirely blunted by phloretin (Fig. 2d).
Substrate transporters and transport in HL-1 cardiomyocytes. a HL-1 cells were transfected with either pCMV (control) or pCMV-GLUT4myc 24 h after seeding. After a further 48 h cells were lysed and the lysates separated by SDS-PAGE and blotted for GLUT4, myc and CD36. Caveolin 3 (Cav3) served as a loading control. Representative western blots are shown. b, c At 48 h after transfection, serum-depleted cells were treated with 200 nmol/l insulin, electric-field stimulation or 1 μmol/l oligomycin for 30 min. Afterwards, plasmalemmal GLUT4myc (b) and CD36 (c) were detected by immunostaining. d, e Serum-depleted cells were incubated with DMSO (control), 0.2 mmol/l phloretin or 0.5 mmol/l SSO for 30 min. Unbound SSO was washed away and cells were stimulated with 200 nmol/l insulin for 30 min in the continued presence of phloretin (where indicated). Afterwards, the cells were incubated with a substrate mix containing 2-deoxy-d-[3H]glucose and [14C]palmitate for 10 min. (d) Glucose uptake and (e) palmitate uptake were quantified by scintillation counting of the cell lysates. The uptake component that was not inhibited by phloretin, i, presents non-protein-mediated uptake (i.e. passive diffusion), and the uptake component that was not inhibited by SSO, ii, presents the component of palmitate uptake that was independent of CD36. Data are mean values ± SEM of three independent experiments (n=3), with triplicate measurements for each condition. *Statistically different from corresponding basal value (p < 0.05)
Abundance of GLUT4myc and CD36 at the plasma membrane after VAMP knockdown
HL-1 cells can efficiently be transfected with siRNAs using lipid-based transfection reagents (Fig. 3a). The transfection of the selected siRNAs directed against VAMP isoforms 2, 3, 4, 5 and 7 led to a protein repression of 81%, 54%, 46%, 62% and 55% (p < 0.05, n=3), respectively, and had no cross-reactivity between the different VAMP isoforms as verified by western blot analysis (Fig. 3b).
Transfection and knockdown of genes encoding VAMP isoforms. a HL-1 cells were transfected with different concentrations of fluorescence-labelled siRNA 24 h after seeding. Transfection efficiency was microscopically determined 6 h after transfection by assessment of the incorporated fluorescence. b HL-1 cells were transfected with siRNAs against VAMP2–8 24 h after seeding. After a further 48 h, cells were lysed and the lysates separated by SDS-PAGE and subsequently blotted for each VAMP isoform. Protein kinase B/AKT (AKT) served as a loading control. Representative western blots are shown. FM, fluorescence microscopy; LM, light microscopy; nc, non-coding; IB, immuno blot
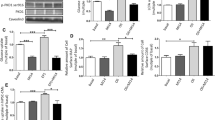
As found with the non-transfected HL-1 cells (Fig. 2), insulin and oligomycin also increased the plasmalemmal amount of GLUT4myc after transfection with a non-coding siRNA, by 1.4- and 1.6-fold, respectively (Fig. 4a,b). Furthermore, plasmalemmal CD36 was increased 1.3-fold and 1.6-fold, respectively (Fig. 4c). After VAMP2 knockdown, basal CD36 presence at the plasma membrane was increased 1.2-fold. Furthermore, the insulin-stimulated increase of both GLUT4myc and CD36 at the plasma membrane was completely inhibited. In contrast, oligomycin-stimulated GLUT4myc and CD36 translocation was not affected. VAMP3 knockdown had no effect on insulin-stimulated transporter translocation but completely inhibited oligomycin-stimulated GLUT4myc and CD36 translocation. Differences between GLUT4myc and CD36 translocation were observed after VAMP4 knockdown. While insulin- and oligomycin-stimulated GLUT4myc translocation remained unaffected, stimulation of CD36 translocation by both compounds was completely inhibited. VAMP5 knockdown increased basal GLUT4myc abundance at the plasma membrane 1.2-fold, while completely inhibiting the stimulatory effects of both insulin and oligomycin on translocation of this transporter. Simultaneously, insulin-stimulated CD36 translocation was completely inhibited, whereas oligomycin-stimulated CD36 translocation was not affected. VAMP7 knockdown increased the basal presence of GLUT4myc at the plasma membrane by 1.5-fold. In spite of the increased basal plasmalemmal content of GLUT4myc in VAMP7-silenced cells, the ability of insulin and oligomycin to stimulate GLUT4myc translocation was largely maintained. In contrast to VAMP5 knockdown, VAMP7 knockdown had no effect on CD36 translocation.
Plasmalemmal abundance of substrate transporters after knockdown of genes encoding VAMP isoforms. a HL-1 cells were co-transfected with VAMP2–7 siRNAs and pCMV-GLUT4myc 32 h after seeding. After a further 28 h the cells were serum depleted for 16 h and subsequently treated with 200 nmol/l insulin or 1 μmol/l oligomycin for 30 min (white bars, basal; grey bars, insulin; black bars, oligomycin). Afterwards, plasmalemmal GLUT4myc (b) and plasmalemmal CD36 (c) were detected by immunostaining. Data are mean values ± SEM of six independent experiments (n=6), with triplicate measurements for each condition. Statistically different from corresponding value in control group (*p < 0.05); statistically different from corresponding basal value († p < 0.05). nc, non-coding
When measuring substrate uptake, changes in the plasmalemmal abundance of GLUT4myc and CD36 generally resulted in concomitant changes in glucose and palmitate uptake (Fig. 5), indicating that the dynamics of substrate utilisation directly correlate with the abundance of both transporters at the plasma membrane. However, the effects of VAMP2 knockdown on insulin-stimulated glucose uptake and of VAMP3 knockdown on oligomycin-stimulated glucose uptake (Fig. 5b) were significantly lower than the effects measured on GLUT4myc translocation (Fig. 4b). Furthermore, VAMP5 knockdown had a significantly greater effect on glucose uptake than on GLUT4myc translocation. To test whether these discrepancies between GLUT4myc translocation and glucose uptake might be due to GLUT1-mediated glucose uptake, we used the specific GLUT4 inhibitor indinavir, which does not interfere with GLUT1-mediated glucose uptake [20]. In non-coding-siRNA-transfected cells, indinavir inhibited basal glucose uptake by 41% (Fig. 6). In VAMP5-silenced cells treated with indinavir basal glucose uptake was elevated by 1.5-fold compared with basal uptake by the indinavir-treated control cells (Fig. 6). These findings indicate that GLUT1 is an important contributor to basal glucose uptake, and that its quantitative involvement is increased upon VAMP5 silencing. However, insulin- or oligomycin-stimulated glucose uptake was completely inhibited by indinavir treatment (Fig. 6), demonstrating that GLUT4 is entirely responsible for the increase in stimulus-induced glucose uptake without any role of GLUT1. Just as in VAMP5-depleted cells in the absence of indinavir (Fig. 5b), indinavir-treated cells lacking VAMP5 did not display altered glucose uptake upon stimulation with insulin or oligomycin (Fig. 6).
Substrate uptake after knockdown of genes encoding VAMP isoforms. a HL-1 cells were transfected with siRNAs 24 h after seeding. After a further 28 h the cells were serum depleted for 16 h and subsequently treated with 200 nmol/l insulin or 1 μmol/l oligomycin for 30 min (white bars, basal; grey bars, insulin; black bars, oligomycin). Afterwards, the cells were incubated with a substrate mix containing 2-deoxy-d-3H-glucose and 14C-palmitate for 10 min. Glucose uptake (b) and palmitate uptake (c) were quantified by scintillation counting of the cell lysates and corrected for the uptake component that could not be inhibited by phloretin (passive diffusion component). Data are mean values ± SEM of six independent experiments (n = 6), with triplicate measurements for each condition. Statistically different from corresponding value in control group (*p < 0.05); statistically different from corresponding basal value († p < 0.05). nc, non-coding
Inhibition of GLUT4-mediated glucose uptake with indinavir. HL-1 cells were transfected with siRNAs 24 h after seeding. After a further 32 h the cells were serum depleted for 16 h and subsequently treated with 200 nmol/l insulin or 1 μmol/l oligomycin for 30 min (white bars, basal; grey bars, insulin; black bars, oligomycin). Afterwards, the cells were incubated with a substrate mix containing 2-deoxy-d-3H-glucose and 14C-palmitate for 10 min, either in the presence or absence of 100 μmol/l indinavir. Glucose uptake into the cells was quantified by scintillation counting of the cell lysates. Data are mean values ± SEM of four independent experiments (n=4), with triplicate measurements for each condition. Statistically different from corresponding value in control group without indinavir (*p < 0.05); statistically different from corresponding basal value († p < 0.05); statistically different from corresponding value in group transfected with non-coding siRNA (‡ p < 0.05). nc, non-coding
Effects of VAMP3 knockdown on electric-field stimulated GLUT4myc and CD36 translocation
As mentioned above, oligomycin was used to increase the intracellular AMP/ATP ratio and activate AMPK signalling. Therefore, the effects of VAMP gene knockdown on oligomycin-stimulated transporter translocation and substrate uptake are expected to be found also in contraction-induced transporter translocation. To verify this, we measured GLUT4myc and CD36 translocation in VAMP3-silenced cells that were contracted using electric-field stimulation (Fig. 7a). As expected, the translocation of both GLUT4myc and CD36 were completely inhibited after VAMP3 knockdown (Fig. 7b, c). Hence, VAMP3 is similarly involved in both oligomycin- and contraction-induced GLUT4myc and CD36 translocation, confirming that both stimuli use the same trafficking machinery to move these transporters to the cell surface. By inference, the effects of knockdown of genes encoding other VAMP isoforms on oligomycin-stimulated transporter translocation can, most likely, be extrapolated to contraction-stimulated GLUT4myc and CD36 translocation.
Contraction-stimulated transporter translocation after VAMP3 knockdown. a HL-1 cells were co-transfected with VAMP3 siRNA and pCMV-GLUT4myc 24 h after seeding. After a further 32 h the cells were serum depleted for 16 h and subsequently treated with electric-field stimulation (2 Hz) for 30 min. Afterwards, plasmalemmal GLUT4myc (b) and plasmalemmal CD36 (c) was detected by immunostaining. Data are mean values ± SEM of three independent experiments (n=3), with triplicate measurements for each condition. Statistically different from corresponding basal value (*p < 0.05). nc, non-coding
Discussion
GLUT4 and CD36 dynamically traffic between subcellular compartments and the plasma membrane in cardiomyocytes. Insulin and contraction trigger the accumulation of both substrate transporters at the plasma membrane to increase the uptake of glucose and LCFA. In the present study, we investigated the involvement of VAMPs in the process of GLUT4 and CD36 translocation to the plasma membrane on stimulation with insulin and increased AMPK activity. We identified myocardial VAMP isoforms that: (1) are shared by the GLUT4 and CD36 trafficking machineries but discriminate between insulin-stimulated and contraction-induced translocation processes; (2) discriminate between CD36 and GLUT4 traffic; and (3) contribute differently to the subcellular distribution of these substrate transporters. Interestingly, it is of note that despite residual levels of VAMP of 20–50%, the inhibitory effects of these gene knockdowns on stimulated transporter translocation were complete. More specifically, we observed no linear correlation between knockdown efficiency of each of the VAMPs and changes in plasmalemmal transporter abundance when comparing the results from the individual knockdown experiments. This indicates that GLUT4 and CD36 trafficking in cardiomyocytes is very sensitive to already small modulations of VAMP abundance.
Selective VAMPs are necessary for both GLUT4 and CD36 translocation, but discriminate between insulin- and contraction-induced translocation
Previous studies showed remarkable similarity between the regulation of GLUT4 and CD36 translocation, especially in the sense that both depend on the same signalling processes (i.e. the phosphoinositide-3 kinase [PI3K]/AKT axis upon insulin stimulation and the LKB1/AMPK axis upon contraction stimulation [1]). The present study further reveals that selected VAMPs (i.e. VAMP2, VAMP3 and VAMP5) are involved in the trafficking of both GLUT4 and CD36. Insulin-stimulated translocation of GLUT4 and CD36 was dependent on VAMP2 and VAMP5. The role of VAMP2 in insulin-stimulated GLUT4 translocation has been well established in skeletal muscle cells and adipocytes [9, 21]. However, the additional involvement of VAMP5 discloses a difference between cardiomyocytes and adipocytes [21]. Still, the function of VAMP5 is rather complex as it is additionally involved in contraction-induced GLUT4 translocation. The mechanism by which insulin signals to VAMP2 and VAMP5 could include PI3K-mediated activation of protein kinase C ζ [22]. The notion that both VAMP2 and VAMP5 are necessary for insulin-stimulated transporter translocation indicates that these VAMPs fulfil non-complementary roles in this process. Perhaps these VAMPs operate at different steps in the translocation process.
In contrast to the findings with VAMP2, we found VAMP3 to be selectively involved in contraction-induced translocation of both GLUT4 and CD36. Previous evidence for a role of VAMP3 in substrate-transporter translocation comes from studies in which VAMP3, just like VAMP2, has been shown to co-localise with GLUT4 [23, 24], whereas this has not yet been determined for CD36. However, functional evidence of the involvement of VAMP3 in contraction-induced transporter translocation is lacking in these previous studies. Our observation that VAMP3 is involved in contraction-induced transporter translocation is, however, difficult to reconcile with a study in rat skeletal muscle in which VAMP2, VAMP5 and VAMP7, but not VAMP3, migrated to the plasma membrane together with GLUT4 upon contraction stimulation [24]. The observation by Rose et al. that the plasmalemmal presence of VAMP3 is not increased upon contraction might be explained by a rapid dissociation of the VAMP3-containing SNARE complex by NSF and SNAP at the plasma membrane [25, 26]. However, tissue differences might also explain these controversial observations.
Taken together, the shared involvement of distinct VAMPs in both GLUT4 and CD36 translocation indicates that CD36 translocation, just like GLUT4 translocation, is a vesicle-mediated process dependent on SNARE-complex formation. Moreover, this shared involvement further emphasises the similarity in the protein machinery regulating both processes.
Selective VAMPs can discriminate between GLUT4 and CD36 translocation
Besides the shared involvement of specific VAMPs in both GLUT4 and CD36 translocation, there are also VAMPs that discriminate between GLUT4 and CD36 translocation. We found VAMP4 to be involved specifically in CD36 translocation induced either by insulin or contraction. Hence, this VAMP isoform is an essential component of the CD36-dedicated trafficking machinery that translocates CD36 to the plasma membrane independently from GLUT4. VAMP5 is specifically involved in contraction-induced translocation of GLUT4, but not CD36. This contrasts with the involvement of VAMP5 in insulin-induced translocation of both transporters and shows that the involvement of VAMP5 in the regulation of transporter translocation is complex. Furthermore, the function of VAMP5 appears to be cell-type specific. While VAMP5 is not involved in GLUT4 trafficking in adipocytes [21], we and others could show its involvement in GLUT4 translocation in contracting myocytes [24].
Selective VAMPs contribute differently to the basal subcellular distribution of substrate transporters
Trafficking proteins are not only involved in stimulus-induced translocation processes, but also in the subcellular retention of a given recycling protein, so that upon termination of the stimulus, this protein returns into its intracellular storage compartment [5]. Hence, VAMPs might not only be involved in the translocation of GLUT4 and CD36 to the plasma membrane but also in their intracellular sorting. A reduction in specific VAMPs could result in an increased basal presence of the substrate transporters at the plasma membrane, either through unregulated dispersion or inhibited internalisation.
The present findings indicate that VAMP5 and VAMP7 mediate basal GLUT4 retention. Interestingly, studies in C2C12 myoblasts have revealed the presence of VAMP5 at the plasma membrane [27]. Combining this finding with the present data suggests that the plasmalemmal presence of VAMP5 is important in GLUT4 internalisation. As we also established that VAMP5 is additionally involved in insulin- and contraction-induced GLUT4 translocation, VAMP5 appears to have a dual function in the regulation of GLUT4 distribution. Furthermore, we observed a discrepancy between GLUT4 abundance at the plasma membrane and glucose uptake in VAMP5-depleted cells: the latter was disproportionally increased. This discrepancy is likely to be attributed to GLUT1, which is the second most important glucose transporter in the heart [28, 29]. Inhibition of GLUT4-mediated glucose uptake by indinavir revealed that the residual glucose uptake (via GLUT1) was increased by 1.5-fold in VAMP5-depleted cells. Therefore, VAMP5 might be involved in GLUT1 internalisation as well as GLUT4 internalisation under basal conditions. Alternatively, plasmalemmal GLUT1 might be upregulated in VAMP5-depeleted cells as a secondary effect to compensate for the loss of plasmalemmal GLUT4. This has already been observed in cardiomyocytes and adipocytes lacking GLUT4 [30, 31]. Furthermore, a compensatory action of GLUT1 might serve as an explanation for the incomplete inhibition of insulin-stimulated glucose uptake in VAMP2-depleted cells and of contraction-stimulated glucose uptake in VAMP3-depleted cells in comparison with complete inhibition of GLUT4 translocation in both cases.
GLUT4, but not CD36, also accumulated at the plasma membrane after VAMP7 knockdown, and even to a markedly greater extent than after VAMP5 knockdown. However, in contrast to VAMP5 knockdown, insulin and oligomycin further increased the sarcolemmal abundance of GLUT4 in VAMP7-depleted cells. Hence, VAMP7 solely functions in GLUT4 internalisation and, unlike VAMP5, has no additional role in stimulus-induced GLUT4 translocation.
We also unveiled that a VAMP isoform—VAMP2—is involved in basal CD36 retention. This ability of VAMP2 to reduce basal levels of CD36 at the plasma membrane is not accompanied by a similar effect of this VAMP isoform on basal GLUT4 retention. Therefore, next to VAMP5 and VAMP7, VAMP2 knockdown also specifically increases the sarcolemmal presence of one of the two transporters under basal conditions. Because we also found VAMP2 to be involved in insulin-stimulated CD36 translocation, this additional involvement in basal CD36 retention points to a dual role of VAMP2 in the regulation of CD36 trafficking. Hence, the dual role of VAMP2 in CD36 trafficking mirrors the dual role of VAMP5 in GLUT4 traffic, and further indicates that both processes are similarly regulated though, in part, by different VAMP isoforms.
Concluding remarks
These findings indicate the potential of VAMPs to serve as targets for the so-called metabolic modulation therapy [32, 33]. For instance, pharmacological strategies to stimulate cardiac glucose uptake via increasing myocardial VAMP2 production or decreasing myocardial VAMP4/VAMP7 production is expected to be beneficial for the diabetic heart in which glucose uptake is repressed in favour of LCFA. Interestingly, VAMP2-silenced HL-1 cells resemble insulin-resistant cardiomyocytes because: (1) use of LCFA is increased already in the basal state; (2) insulin has lost the ability to stimulate LCFA and glucose uptake; but (3) contraction-induced glucose and LCFA uptake are unimpaired. Similar changes occur in the cardiac and skeletal muscle of insulin-resistant obese Zucker rats [2, 34]. Furthermore, VAMP2 production is decreased in the retinas from streptozotocin-diabetic rats [35] and in insulin-secreting cells after 72 h exposure to palmitate [36]. Whether inhibition of VAMP2 also takes place in the diabetic heart remains to be determined.
Abbreviations
- (p)ACC:
-
(phospho-)Acetyl-coA carboxylase
- (p)AMPK:
-
(phospho-)AMP-activated protein kinase
- pCMV:
-
Mammalian expression vector containing the human cytomegalovirus promoter
- LCFA:
-
Long-chain fatty acid
- PI3K:
-
Phosphoinositide-3 kinase
- PKB/AKT:
-
Protein kinase B
- Q-SNARE:
-
SNARE with glutamine in SNARE motif
- R-SNARE:
-
SNARE with arginine in SNARE motif
- siRNA:
-
Small interfering RNA
- SNAP:
-
Soluble NSF attachment protein
- SNARE:
-
Soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- SSO:
-
Sulfo-N-succinimidyloleate
- t-SNARE:
-
Target-membrane-associated SNARE
- VAMP:
-
Vesicle-associated membrane protein
- v-SNARE:
-
Vesicle-associated SNARE
References
Schwenk RW, Luiken JJ, Bonen A, Glatz JF (2008) Regulation of sarcolemmal glucose and fatty acid transporters in cardiac disease. Cardiovasc Res 79:249–258
Coort SL, Hasselbaink DM, Koonen DP et al (2004) Enhanced sarcolemmal FAT/CD36 content and triacylglycerol storage in cardiac myocytes from obese Zucker rats. Diabetes 53:1655–1663
Russell LK, Finck BN, Kelly DP (2005) Mouse models of mitochondrial dysfunction and heart failure. J Mol Cell Cardiol 38:81–91
Ouwens DM, Diamant M, Fodor M et al (2007) Cardiac contractile dysfunction in insulin-resistant rats fed a high-fat diet is associated with elevated CD36-mediated fatty acid uptake and esterification. Diabetologia 50:1938–1948
Hong W (2005) SNAREs and traffic. Biochim Biophys Acta 1744:493–517
Sollner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE (1993) A protein assembly–disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell 75:409–418
Trimble WS, Cowan DM, Scheller RH (1988) VAMP-1: a synaptic vesicle-associated integral membrane protein. Proc Natl Acad Sci USA 85:4538–4542
Zeng Q, Subramaniam VN, Wong SH et al (1998) A novel synaptobrevin/VAMP homologous protein (VAMP5) is increased during in vitro myogenesis and present in the plasma membrane. Mol Biol Cell 9:2423–2437
Randhawa VK, Bilan PJ, Khayat ZA et al (2000) VAMP2, but not VAMP3/cellubrevin, mediates insulin-dependent incorporation of GLUT4 into the plasma membrane of L6 myoblasts. Mol Biol Cell 11:2403–2417
Kawanishi M, Tamori Y, Okazawa H, Araki S, Shinoda H, Kasuga M (2000) Role of SNAP23 in insulin-induced translocation of GLUT4 in 3T3-L1 adipocytes. Mediation of complex formation between syntaxin4 and VAMP2. J Biol Chem 275:8240–8247
Randhawa VK, Thong FS, Lim DY et al (2004) Insulin and hypertonicity recruit GLUT4 to the plasma membrane of muscle cells by using N-ethylmaleimide-sensitive factor-dependent SNARE mechanisms but different v-SNAREs: role of TI-VAMP. Mol Biol Cell 15:5565–5573
Luiken JJ, Coort SL, Willems J et al (2003) Contraction-induced fatty acid translocase/CD36 translocation in rat cardiac myocytes is mediated through AMP-activated protein kinase signaling. Diabetes 52:1627–1634
Wang Q, Khayat Z, Kishi K, Ebina Y, Klip A (1998) GLUT4 translocation by insulin in intact muscle cells: detection by a fast and quantitative assay. FEBS Lett 427:193–197
van Oort MM, van Doorn JM, Bonen A et al (2008) Insulin-induced translocation of CD36 to the plasma membrane is reversible and shows similarity to that of GLUT4. Biochim Biophys Acta 1781:61–71
Shuralyova I, Tajmir P, Bilan PJ, Sweeney G, Coe IR (2004) Inhibition of glucose uptake in murine cardiomyocyte cell line HL-1 by cardioprotective drugs dilazep and dipyridamole. Am J Physiol 286:H627–H632
Palanivel R, Eguchi M, Shuralyova I, Coe I, Sweeney G (2006) Distinct effects of short- and long-term leptin treatment on glucose and fatty acid uptake and metabolism in HL-1 cardiomyocytes. Metabolism 55:1067–1075
Luiken JJ, Willems J, van der Vusse GJ, Glatz JF (2001) Electrostimulation enhances FAT/CD36-mediated long-chain fatty acid uptake by isolated rat cardiac myocytes. Am J Physiol Endocrinol Metab 281:E704–E712
McNeill-Blue C, Wetmore BA, Sanchez JF, Freed WJ, Merrick BA (2006) Apoptosis mediated by p53 in rat neural AF5 cells following treatment with hydrogen peroxide and staurosporine. Brain Res 1112:1–15
Luiken JJ, van Nieuwenhoven FA, America G, van der Vusse GJ, Glatz JF (1997) Uptake and metabolism of palmitate by isolated cardiac myocytes from adult rats: involvement of sarcolemmal proteins. J Lipid Res 38:745–758
Rudich A, Konrad D, Torok D et al (2003) Indinavir uncovers different contributions of GLUT4 and GLUT1 towards glucose uptake in muscle and fat cells and tissues. Diabetologia 46:649–658
Williams D, Pessin JE (2008) Mapping of R-SNARE function at distinct intracellular GLUT4 trafficking steps in adipocytes. J Cell Biol 180:375–387
Braiman L, Alt A, Kuroki T et al (2001) Activation of protein kinase C zeta induces serine phosphorylation of VAMP2 in the GLUT4 compartment and increases glucose transport in skeletal muscle. Mol Cell Biol 21:7852–7861
Dugani CB, Randhawa VK, Cheng AW, Patel N, Klip A (2008) Selective regulation of the perinuclear distribution of glucose transporter 4 (GLUT4) by insulin signals in muscle cells. Eur J Cell Biol 87:337–351
Rose AJ, Jeppesen J, Kiens B, Richter EA (2009) Effects of contraction on localization of GLUT4 and v-SNARE isoforms in rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol 297:R1228–1237
Bar-On D, Winter U, Nachliel E et al (2008) Imaging the assembly and disassembly kinetics of cis-SNARE complexes on native plasma membranes. FEBS Lett 582:3563–3568
Winter U, Chen X, Fasshauer D (2009) A conserved membrane attachment site in α-SNAP facilitates NSF-driven SNARE complex disassembly. J Biol Chem 284:31817–31826
Zeng Q, Tran TT, Tan HX, Hong W (2003) The cytoplasmic domain of Vamp4 and Vamp5 is responsible for their correct subcellular targeting: the N-terminal extenSion of VAMP4 contains a dominant autonomous targeting signal for the trans-Golgi network. J Biol Chem 278:23046–23054
Becker C, Sevilla L, Tomas E, Palacin M, Zorzano A, Fischer Y (2001) The endosomal compartment is an insulin-sensitive recruitment site for GLUT4 and GLUT1 glucose transporters in cardiac myocytes. Endocrinology 142:5267–5276
Fischer Y, Thomas J, Sevilla L et al (1997) Insulin-induced recruitment of glucose transporter 4 (GLUT4) and GLUT1 in isolated rat cardiac myocytes. Evidence of the existence of different intracellular GLUT4 vesicle populations. J Biol Chem 272:7085–7092
Katz EB, Stenbit AE, Hatton K, DePinho R, Charron MJ (1995) Cardiac and adipose tissue abnormalities but not diabetes in mice deficient in GLUT4. Nature 377:151–155
Stenbit AE, Katz EB, Chatham JC et al (2000) Preservation of glucose metabolism in hypertrophic GLUT4-null hearts. Am J Physiol 279:H313–H318
Taegtmeyer H, Woods A (2003) Clinical trials report. Metabolic modulation as a principle for myocardial protection. Curr Hypertens Rep 5:443–444
Glatz JF, Bonen A, Ouwens DM, Luiken JJ (2006) Regulation of sarcolemmal transport of substrates in the healthy and diseased heart. Cardiovasc Drugs Ther 20:471–476
Luiken JJ, Arumugam Y, Dyck DJ et al (2001) Increased rates of fatty acid uptake and plasmalemmal fatty acid transporters in obese Zucker rats. J Biol Chem 276:40567–40573
VanGuilder HD, Brucklacher RM, Patel K, Ellis RW, Freeman WM, Barber AJ (2008) Diabetes downregulates presynaptic proteins and reduces basal synapsin I phosphorylation in rat retina. Eur J Neurosci 28:1–11
Lovis P, Roggli E, Laybutt DR et al (2008) Alterations in microRNA expression contribute to fatty acid-induced pancreatic beta-cell dysfunction. Diabetes 57:2728–2736
Acknowledgements
This study was supported by the European Community (Integrated Project LSHM-CT-2004-005272, Exgenesis), the transnational University Limburg, the Natural Sciences and Engineering Research Council of Canada, and the Heart and Stroke Foundation of Ontario. A. Bonen is Canada Research Chair in Metabolism and Health.
Duality of interest
The authors report that there is no duality of interest associated with this manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Schwenk, R.W., Dirkx, E., Coumans, W.A. et al. Requirement for distinct vesicle-associated membrane proteins in insulin- and AMP-activated protein kinase (AMPK)-induced translocation of GLUT4 and CD36 in cultured cardiomyocytes. Diabetologia 53, 2209–2219 (2010). https://doi.org/10.1007/s00125-010-1832-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-010-1832-7