Abstract
Rationale
Drug addiction is a disease with a genetic component that may be involved in different stages of its progression. Cocaine users escalate unit doses and frequency of self-administration events in naturalistic settings. Rats that self-administer drugs of abuse over extended sessions increase the number of infusions over days.
Objectives
Comparison of two genetically different inbred rat strains, Fischer and Lewis, in a new self-administration paradigm whereby rats select between different unit doses of cocaine, thus potentially escalating the unit dose and the number of infusions.
Methods
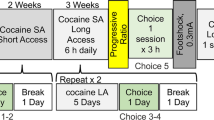
Extended (18 h/day) self-administration sessions lasted for 14 days. Rats had access to two active levers associated with two different unit doses of cocaine. If a rat showed preference for the higher unit dose, then the available doses were escalated in the following session. Four cocaine unit doses were available (0.2, 0.5, 1.25, and 2.5 mg/kg/infusion).
Results
Lewis rats showed a clear preference for the two higher doses of cocaine (70% of rats), with a high percentage (35%) of the individuals escalating to the highest unit dose, and escalated the total amount of cocaine taken over days. Fischer rats, however, preferred the two lower doses (63%) and did not escalate the amount of cocaine taken over days. Fischer, but not Lewis, rats showed an activated hypothalamic–pituitary–adrenal axis in acute withdrawal (24 h).
Conclusion
This work shows the power of a model of extended-access self-administration that allows for the subject-controlled dose-escalation of the unit dose of cocaine, and underlines the genetic differences that modulate cocaine intake.





Similar content being viewed by others
References
Ahmed SH, Koob GF (1998) Transition from moderate to excessive drug intake: change in hedonic set point. Science 282:298–300
Ahmed SH, Koob GF (1999) Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology (Berl) 146:303–312
Ambrosio E, Goldberg SR, Elmer GI (1995) Behavior genetic investigation of the relationship between spontaneous locomotor activity and the acquisition of morphine self-administration behavior. Behav Pharmacol 6:229–237
Anderson KG, Woolverton WL (2005) Effects of clomipramine on self-control choice in Lewis and Fischer 344 rats. Pharmacol Biochem Behav 80:387–393
Cadoni C, Di Chiara G (2007) Differences in dopamine responsiveness to drugs of abuse in the nucleus accumbens shell and core of Lewis and Fischer 344 rats. J Neurochem 103:487–499
Cadoni C, Muto T, Di Chiara G (2009) Nicotine differentially affects dopamine transmission in the nucleus accumbens shell and core of Lewis and Fischer 344 rats. Neuropharmacology 57:496–501
Caine SB, Negus SS, Mello NK, Patel S, Bristow L, Kulagowski J, Vallone D, Saiardi A, Borrelli E (2002) Role of dopamine D2-like receptors in cocaine self-administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists. J Neurosci 22:2977–2988
Calabrese EJ (2008) U-shaped dose response in behavioral pharmacology: historical foundations. Crit Rev Toxicol 38:591–598
Camp DM, Browman KE, Robinson TE (1994) The effects of methamphetamine and cocaine on motor behavior and extracellular dopamine in the ventral striatum of Lewis versus Fischer 344 rats. Brain Res 668:180–193
Chaouloff F, Kulikov A, Sarrieau A, Castanon N, Mormede P (1995) Male Fischer 344 and Lewis rats display differences in locomotor reactivity, but not in anxiety-related behaviours: relationship with the hippocampal serotonergic system. Brain Res 693:169–178
Covington HE 3rd, Tropea TF, Rajadhyaksha AM, Kosofsky BE, Miczek KA (2008) NMDA receptors in the rat VTA: a critical site for social stress to intensify cocaine taking. Psychopharmacology (Berl) 197:203–216
Dhabhar FS, McEwen BS, Spencer RL (1993) Stress response, adrenal steroid receptor levels and corticosteroid-binding globulin levels—a comparison between Sprague-Dawley, Fischer 344 and Lewis rats. Brain Res 616:89–98
Dhabhar FS, McEwen BS, Spencer RL (1997) Adaptation to prolonged or repeated stress-comparison between rat strains showing intrinsic differences in reactivity to acute stress. Neuroendocrinology 65:360–368
Flores G, Wood GK, Barbeau D, Quirion R, Srivastava LK (1998) Lewis and Fischer rats: a comparison of dopamine transporter and receptors levels. Brain Res 814:34–40
Freeman KB, Kearns DN, Kohut SJ, Riley AL (2009) Strain differences in patterns of drug-intake during prolonged access to cocaine self-administration. Behav Neurosci 123:156–164
Garcia-Lecumberri C, Torres I, Marti NS, Crespo JA, Miguens M, Nicanor C, Higuera-Matas A, Ambrosio E (2010) Strain differences in the dose-response relationship for morphine self-administration and impulsive choice between Lewis and Fischer 344 rats. J Psychopharmacol. doi:10.1177/0269881110367444
George FR, Goldberg SR (1989) Genetic approaches to the analysis of addiction processes. Trends Pharmacol Sci 10:78–83
Griffin AC, Whitacre CC (1991) Sex and strain differences in the circadian rhythm fluctuation of endocrine and immune function in the rat: implications for rodent models of autoimmune disease. J Neuroimmunol 35:53–64
Guitart X, Beitner-Johnson D, Marby DW, Kosten TA, Nestler EJ (1992) Fischer and Lewis rat strains differ in basal levels of neurofilament proteins and their regulation by chronic morphine in the mesolimbic dopamine system. Synapse 12:242–253
Haile CN, Hiroi N, Nestler EJ, Kosten TA (2001) Differential behavioral responses to cocaine are associated with dynamics of mesolimbic dopamine proteins in Lewis and Fischer 344 rats. Synapse 41:179–190
Haney M, Spealman R (2008) Controversies in translational research: drug self-administration. Psychopharmacology (Berl) 199:403–419
Hedrich HJ (2006) Taxonomy and Stocks and Strains. In: Suckow MA, Weisbroth SH, Franklin CL (eds) The Laboratory Rat (American College of Laboratory Animal Medicine), 2nd edn. Academic Press, London, pp 71–92
Horan B, Smith M, Gardner EL, Lepore M, Ashby CR Jr (1997) (-)-Nicotine produces conditioned place preference in Lewis, but not Fischer 344 rats. Synapse 26:93–94
Koob G, Kreek MJ (2007) Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry 164:1149–1159
Koob GF, Le Moal M (1997) Drug abuse: hedonic homeostatic dysregulation. Science 278:52–58
Koob GF, Le Moal M (2008a) Addiction and the brain antireward system. Annu Rev Psychol 59:29–53
Koob GF, Le Moal M (2008b) Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci 363:3113–3123
Koob GF, Volkow ND (2010) Neurocircuitry of Addiction. Neuropsychopharmacology 35:217–238
Kosten TA, Ambrosio E (2002) HPA axis function and drug addictive behaviors: insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrinology 27:35–69
Kosten TA, Miserendino MJ, Chi S, Nestler EJ (1994) Fischer and Lewis rat strains show differential cocaine effects in conditioned place preference and behavioral sensitization but not in locomotor activity or conditioned taste aversion. J Pharmacol Exp Ther 269:137–144
Kosten TA, Miserendino MJ, Haile CN, DeCaprio JL, Jatlow PI, Nestler EJ (1997) Acquisition and maintenance of intravenous cocaine self-administration in Lewis and Fischer inbred rat strains. Brain Res 778:418–429
Kosten TA, Zhang XY, Haile CN (2007) Strain differences in maintenance of cocaine self-administration and their relationship to novelty activity responses. Behav Neurosci 121:380–388
Kreek MJ, Schlussman SD, Reed B, Zhang Y, Nielsen DA, Levran O, Zhou Y, Butelman ER (2009) Bidirectional translational research: Progress in understanding addictive diseases. Neuropharmacology 56(Suppl 1):32–43
Kruzich PJ, Chen AC, Unterwald EM, Kreek MJ (2003) Subject-regulated dosing alters morphine self-administration behavior and morphine-stimulated [35S]GTPgammaS binding. Synapse 47:243–249
Mantsch JR, Ho A, Schlussman SD, Kreek MJ (2001) Predictable individual differences in the initiation of cocaine self-administration by rats under extended-access conditions are dose-dependent. Psychopharmacology (Berl) 157:31–39
Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ (2003) Neuroendocrine alterations in a high-dose, extended-access rat self-administration model of escalating cocaine use. Psychoneuroendocrinology 28:836–862
Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ (2004) Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology (Berl) 175:26–36
Mello NK, Negus SS (1996) Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology 14:375–424
Nestler EJ (1992) Molecular mechanisms of drug addiction [published erratum appears in J Neurosci 1992 Aug; 12(8):following table of contents]. J Neurosci 12:2439–2450
Quadros IM, Miczek KA (2009) Two modes of intense cocaine bingeing: increased persistence after social defeat stress and increased rate of intake due to extended access conditions in rats. Psychopharmacology (Berl) 206:109–120
Roth ME, Carroll ME (2004) Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav 78:199–207
Sanchez-Cardoso P, Higuera-Matas A, Martin S, Miguens M, Del Olmo N, Garcia-Lecumberri C, Ambrosio E (2009) Strain differences between Lewis and Fischer 344 rats in the modulation of dopaminergic receptors after morphine self-administration and during extinction. Neuropharmacology 57:8–17
Schlussman SD, Zhou Y, Bailey A, Ho A, Kreek MJ (2005) Steady-dose and escalating-dose “binge” administration of cocaine alter expression of behavioral stereotypy and striatal preprodynorphin mRNA levels in rats. Brain Res Bull 67:169–175
Suzuki T, George FR, Meisch RA (1988) Differential establishment and maintenance of oral ethanol reinforced behavior in Lewis and Fischer 344 inbred rat strains. J Pharmacol Exp Ther 245:164–170
Tornatzky W, Miczek KA (2000) Cocaine self-administration “binges”: transition from behavioral and autonomic regulation toward homeostatic dysregulation in rats. Psychopharmacology (Berl) 148:289–298
Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, True W, Lin N, Toomey R, Eaves L (1998) Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry 55:967–972
Tsuang MT, Lyons MJ, Harley RM, Xian H, Eisen S, Goldberg J, True WR, Faraone SV (1999) Genetic and environmental influences on transitions in drug use. Behav Genet 29:473–479
Wee S, Specio SE, Koob GF (2007) Effects of dose and session duration on cocaine self-administration in rats. J Pharmacol Exp Ther 320:1134–1143
Zernig G, Ahmed SH, Cardinal RN, Morgan D, Acquas E, Foltin RW, Vezina P, Negus SS, Crespo JA, Stockl P, Grubinger P, Madlung E, Haring C, Kurz M, Saria A (2007) Explaining the escalation of drug use in substance dependence: models and appropriate animal laboratory tests. Pharmacology 80:65–119
Zhou Y, Spangler R, LaForge KS, Maggos CE, Ho A, Kreek MJ (1996) Corticotropin-releasing factor and type 1 corticotropin-releasing factor receptor messenger RNAs in rat brain and pituitary during “binge”-pattern cocaine administration and chronic withdrawal. J Pharmacol Exp Ther 279:351–358
Zhou Y, Spangler R, Schlussman SD, Ho A, Kreek MJ (2003) Alterations in hypothalamic-pituitary-adrenal axis activity and in levels of proopiomelanocortin and corticotropin-releasing hormone-receptor 1 mRNAs in the pituitary and hypothalamus of the rat during chronic ‘binge’ cocaine and withdrawal. Brain Res 964:187–199
Acknowledgements
This work was supported by grants from NIH-NIDA P60-DA05130 (MJK) and The Arcadia Charitable Trust (MJK).
RP, AH, ERB, and MJK declare that, except for income received from the primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service, and that there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 481 kb)
Rights and permissions
About this article
Cite this article
Picetti, R., Ho, A., Butelman, E.R. et al. Dose preference and dose escalation in extended-access cocaine self-administration in Fischer and Lewis rats. Psychopharmacology 211, 313–323 (2010). https://doi.org/10.1007/s00213-010-1899-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-010-1899-3