Abstract
Rationale
Oxytocin (OXT) has been proposed as a potential therapeutic agent for post-traumatic stress disorder (PTSD).
Objectives
We aimed to verify whether pharmacological manipulation of the brain OXT system affects cued fear conditioning and fear extinction.
Methods
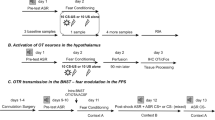
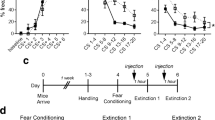
Male rats and mice were intracerebroventricularly administered synthetic OXT (rats, 0.1 or 1.0 μg/5 μl; mice, 0.1 or 0.5 μg/2 μl) and/or an OXT receptor antagonist (OXTR-A; rats, 0.75 μg/5 μl) either prior to fear conditioning or extinction training.
Results
Preconditioning administration of OXT did not affect fear conditioning in rats, but decreased fear expression and facilitated fear extinction. In contrast, preconditioning blockade of OXT neurotransmission by OXTR-A did not affect fear conditioning or fear expression, but impaired fear extinction. When administered before extinction training, OXT impaired fear extinction in both rats and mice, indicating that the effects of OXT on fear extinction are conserved across species. This impairment was OXTR-mediated, as the inhibitory effect of OXT on fear extinction was abolished by prior treatment with OXTR-A. The impaired fear extinction was not a result of reduced locomotion in rats, whereas an apparent decrease in fear expression and facilitation of fear extinction with the higher OXT dose in mice was the result of behavioral hyperactivity.
Conclusions
These results suggest that increasing OXT neurotransmission during traumatic events is likely to prevent the formation of aversive memories. In contrast, OXT treatment before fear extinction training, which would be the comparable timepoint for psychotherapy in PTSD patients, rather delays fear extinction and, therefore, caution is needed before recommending OXT for the treatment of PTSD.





Similar content being viewed by others
References
Barrett D, Gonzalez-Lima F (2004) Behavioral effects of metyrapone on Pavlovian extinction. Neurosci Lett 371(2–3):91–96
Bisson JI, Ehlers A, Matthews R, Pilling S, Richards D, Turner S (2007) Psychological treatments for chronic post-traumatic stress disorder. Systematic review and meta-analysis. Br J Psychiatry 190:97–104
Blume A, Bosch OJ, Miklos S, Torner L, Wales L, Waldherr M, Neumann ID (2008) Oxytocin reduces anxiety via ERK 1/2 activation: local effect within the rat hypothalamic paraventricular nucleus. Eur J Neurosci 27:1947–1956
Bohus B, Kovacs GL, de Wied D (1978) Oxytocin, vasopressin and memory: opposite effects on consolidation and retrieval processes. Brain Res 157:414–417
Bosch OJ, Neumann ID (2008) Brain vasopressin is an important regulator of maternal behavior independent of dams' trait anxiety. Proc Natl Acad Sci 105(44):17139–17144
Brunello N, Davidson JR, Deahl M, Kessler RC, Mendlewicz J, Racagni G, Shalev AY, Zohar J (2001) Posttraumatic stress disorder: diagnosis and epidemiology, comorbidity and social consequences, biology and treatment. Neuropsychobiology 43:150–162
Cammarota M, Bevilaqua LR, Vianna MR, Medina JH, Izquierdo I (2007) The extinction of conditioned fear: structural and molecular basis and therapeutic use. Rev Bras Psiquiatr 29(1):80–85
Carter CS, Altemus M, Chrousos GP (2001) Neuroendocrine and emotional changes in the post-partum period. Prog Brain Res 133:241–249
Condés-Lara M, Rojas-Piloni G, Martínez-Lorenzana G, López-Hidalgo M, Rodríguez-Jiménez J (2009) Hypothalamospinal oxytocinergic antinociception is mediated by GABAergic and opiate neurons that reduce A-delta and C fiber primary afferent excitation of spinal cord cells. Brain Res 1247:38–49
Conrad CD, Galea LAM, Kuroda Y, McEwen BS (1996) Chronic stress impairs spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci 110:1321–1334
Cordero MI, Sandi C (1998) A role for brain glucocorticoid receptors in contextual fear conditioning: dependence upon training intensity. Brain Res 786(1–2):11–17
Cordero MI, Kruyt ND, Merino JJ, Sandi C (2002) Glucocorticoid involvement in memory formation in a rat model for traumatic memory. Stress 5(1):73–79
Davidson JR, Stein DJ, Shalev AY, Yehuda R (2004) Posttraumatic stress disorder: acquisition, recognition, course, and treatment. J Neuropsychiatry Clin Neurosci 16:135–147
de Kloet ER, Oitzl MS, Joels M (1999) Stress and cognition, are corticosteroids good or bad guys? Trends Neurosci 22:422–426
de Wied D, Elands J, Kovacs G (1991) Interactive effects of neurohypophyseal neuropeptides with receptor antagonists on passive avoidance behavior: mediation by a cerebral neurohypophyseal hormone receptor. Proc Natl Acad Sci USA 88:1494–1498
Delanoy RL, Dunn AJ, Walter R (1979) Neurohypophyseal hormones and behavior: effects of intracerebroventricularly injected hormone analogs in mice. Life Sci 24:651–658
Dilger S, Straube T, Mentzel HJ, Fitzek C, Reichenbach JR, Hecht H, Krieschel S, Gutberlet I, Miltner WH (2003) Brain activation to phobia-related pictures in spider phobic humans: an event-related functional magnetic resonance imaging study. Neurosci Lett 348:29–32
Ebner K, Bosch OJ, Krömer SA, Singewald N, Neumann ID (2005) Release of oxytocin in the rat central amygdala modulates stress-coping behavior and the release of excitatory amino acids. Neuropsychopharmacol 30(2):223–230
Fanselow MS (1980) Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci 15(4):177–182
Fleshner M, Pugh CR, Tremblay D, Rudy JW (1997) DHEA-S selectively impairs contextual-fear conditioning: support for the antiglucocorticoid hypothesis. Behav Neurosci 111(3):512–517
Gülpinar MA, Yegen BC (2004) The physiology of learning and memory: role of peptides and stress. Curr Protein Pept Sci 5(6):457–473
Heinrichs M, Meinlschmidt G, Neumann ID, Wagner S, Kirschbaum C, Ehlert U, Hellhammer DH (2001) Effects of suckling on hypothalamic–pituitary–adrenal axis responses to psychosocial stress in postpartum lactating women. J Clin Endocrinol Metab 86:4798–4804
Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U (2003) Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry 54:1389–1398
Herry C, Trifilieff P, Micheau J, Luthi A, Mons N (2006) Extinction of auditory fear conditioning requires MAPK/ERK activation in the basolateral amygdala. Eur J Neurosci 24:261–269
Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A (2008) Switching on and off fear by distinct neuronal circuits. Nature 454:600–606
Ipser J, Seedat S, Stein DJ (2006) Pharmacotherapy for post-traumatic stress disorder—a systematic review and meta-analysis. S Afr Med J 96:1088–1096
Kaidanovich-Beilin O, Lipina TV, Takao K, van Eede M, Hattori S, Laliberté C, Khan M, Okamoto K, Chambers JW, Fletcher PJ, MacAulay K, Doble BW, Henkelman M, Miyakawa T, Roder J, Woodgett JR (2009) Abnormalities in brain structure and behavior in GSK-3alpha mutant mice. Mol Brain 19:2–35
Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A (2005) Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci 25(49):11489–11493
Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH (1996) Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sci 58:1475–1483
Kovács GL, Bohus B, Versteeg DHG, de Kolet ER, de Wied D (1979) Effect of oxytocin and vasopressin on memory consolidation: sites of action and catecholaminergic correlates after local microinjection into limbic–midbrain structures. Brain Res 175:303–314
Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, Stout JC, Nathan PJ (2010) Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacol 35(12):2403–2413
Landgraf R, Neumann ID (2004) Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol 25:150–176
Lattal KM, Abel T (2001) Different requirements for protein synthesis in acquisition and extinction of spatial preferences and context-evoked fear. J Neurosci 21(15):5773–5780
LeDoux JE, Iwata J, Cicchetti P, Reis DJ (1988) Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci 8:2517–2529
Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Paré D (2008) Amygdala intercalated neurons are required for expression of fear extinction. Nature 454:642–645
Loscertales M, Rose SP, Sandi C (1997) The corticosteroid synthesis inhibitors metyrapone and aminoglutethimide impair long-term memory for a passive avoidance task in day-old chicks. Brain Res 769(2):357–361
Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID (2011) The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacol 36(11):2159–2168
Lundeberg T, Uvnäs-Moberg K, Agren G, Bruzelius G (1994) Anti-nociceptive effects of oxytocin in rats and mice. Neurosci Lett 28;170(1):153–157
Lupien SJ, McEwen BS (1997) The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Rev 24:1–27
Makkar SR, Zhang SQ, Cranney J (2010) Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory. Neuropsychopharmacol 35:1625–1652
Manning M, Stoev S, Chini B, Durroux T, Mouillac B, Guillon G (2008) Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT receptors: research tools and potential therapeutic agents. Prog Brain Res 170:473–512
Marshall RD, Pierce D (2000) Implications of recent findings in posttraumatic stress disorder and the role of pharmacotherapy. Harv Rev Psychiatry 7(5):247–256
McCarthy MM, McDonald CH, Brooks PJ, Goldman D (1996) An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiol Behav 60(5):1209–1215
Meisenberg G, Simmons WH (1982) Behavioral effects of intracerebroventricularly administered neurohypophyseal hormone analogs in mice. Pharmac Biochem Behav 16:819–825
Muigg P, Hetzenauer A, Hauer G, Hauschild M, Gaburro S, Frank E, Landgraf R, Singewald N (2008) Impaired extinction of learned fear in rats selectively bred for high anxiety—evidence of altered neuronal processing in prefrontal–amygdala pathways. Eur J Neurosci 28(11):2299–2309
Myers KM, Davis M (2002) Behavioral and neural analysis of extinction. Neuron 36(4):567–584
Neumann ID (2007) Stimuli and consequences of dendritic release of oxytocin within the brain. Biochem Soc Trans 35(5):1252–1257
Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R (2000) Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus. J Neuroendocrinol 12(3):235–243
Olff M, Langeland W, Witteveen A, Denys D (2010) A psychobiological rationale for oxytocin in the treatment of posttraumatic stress disorder. CNS Spectr 15(8):522–530
Quirk GJ, Likhtik E, Pelletier JG, Paré D (2003) Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci 23:8800–8807
Quirk GJ, Paré D, Richardson R, Herry C, Monfils MH, Schiller D, Vicentic A (2010) Erasing fear memories with extinction training. J Neurosci 30(45):14993–14997
Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK (2000) Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry 47:769–776
Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, Schechter LE, Rizzo S, Rahman Z, Rosenzweig-Lipson S (2006) Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology (Berl) 185(2):218–225
Roozendaal B, Schoorlemmer GH, Wiersma A, Sluyter S, Driscoll P, Koolhaas JM, Bohus B (1992) Opposite effects of central amygdaloid vasopressin and oxytocin on the regulation of conditioned stress responses in male rats. Ann NY Acad Sci 652:460–461
Sandi C (1998) Role and mechanisms of action of glucocorticoids in memory formation. Neural Plasticity 6:39–49
Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK (2004) Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry 61:168–176
Slattery DA, Neumann ID (2008) No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. J Physiol 586(2):377–385
Slattery DA, Uschold N, Magoni M, Bär J, Popoli M, Neumann ID, Reber SO (2012) Behavioural consequences of two chronic psychosocial stress paradigms: anxiety without depression. Psychoneuroendocrinol 37(5):702–714
Sotres-Bayon F, Bush DE, LeDoux JE (2007) Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacol 32:1929–1940
Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG (2002) Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry 59:1027–1034
Stein DJ, Ipser JC, Seedat S (2006) Pharmacotherapy for post traumatic stress disorder (PTSD). Cochrane Database Syst Rev (1):CD002795
Stein DJ, Ipser J, McAnda N (2009) Pharmacotherapy of posttraumatic stress disorder: a review of meta-analyses and treatment guidelines. CNS Spectr 14:25–31
Takao K, Tanda K, Nakamura K, Kasahara J, Nakao K, Katsuki M, Nakanishi K, Yamasaki N, Toyama K, Adachi M, Umeda M, Araki T, Fukunaga K, Kondo H, Sakagami H, Miyakawa T (2010) Comprehensive behavioral analysis of calcium/calmodulin-dependent protein kinase IV knockout mice. PLoS One 5(3):e9460
Toth I, Dietz M, Peterlik D, Huber SE, Fendt M, Neumann ID, Flor PJ, Slattery DA (2012a) Pharmacological interference with metabotropic glutamate receptor subtype 7 but not subtype 5 differentially affects within- and between-session extinction of Pavlovian conditioned fear. Neuropharmacol 62(4):1619–1626
Toth I, Neumann ID, Slattery DA (2012b) Social fear conditioning: a novel and specific animal model to study social anxiety disorder. Neuropsychopharmacology. doi:10.1038/npp.2011.329
Uvnäs-Moberg K, Bruzelius G, Alster P, Bileviciute I, Lundeberg T (1992) Oxytocin increases and a specific oxytocin antagonist decreases pain threshold in male rats. Acta Physiol Scand 144(4):487–488
Uvnäs-Moberg K, Ahlenius S, Hillegaart V, Alster P (1994) High doses of oxytocin cause sedation and low doses cause an anxiolytic-like effect in male rats. Pharmacol Biochem Behav 49(1):101–106
Viero C, Shibuya I, Kitamura N, Verkhratsky A, Fujihara H, Katoh A, Ueta Y, Zingg HH, Chvatal A, Sykova E, Dayanithi G (2010) Oxytocin: crossing the bridge between basic science and pharmacotherapy. CNS Neurosci Ther 16(5):138–156
Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, Magara F, Stoop R (2011) Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science 333(6038):104–107
Waldherr M, Neumann ID (2007) Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc Natl Acad Sci USA 104(42):16681–16684
Weeber EJ, Atkins CM, Selcher JC, Varga AW, Mirnikjoo B, Paylor R, Leitges M, Sweatt JD (2000) A role for the beta isoform of protein kinase C in fear conditioning. J Neurosci 20(16):5906–5914
Windle RJ, Shanks N, Lightman SL, Ingram CD (1997) Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinol 138:2829–2834
Wotjak CT, Landgraf R, Engelmann M (2008) Listening to neuropeptides by microdialysis: echoes and new sounds? Pharmacol Biochem Behav 90(2):125–134
Yang YL, Chao PK, Lu KT (2006) Systemic and intra-amygdala administration of glucocorticoid agonist and antagonist modulate extinction of conditioned fear. Neuropsychopharmacol 31(5):912–924
Yang YL, Chao PK, Ro LS, Wo YY, Lu KT (2007) Glutamate NMDA receptors within the amygdala participate in the modulatory effect of glucocorticoids on extinction of conditioned fear in rats. Neuropsychopharmacol 32(5):1042–1051
Yang J, Liang JY, Zhang XY, Qiu PY, Pan YJ, Li P, Zhang J, Hao F, Wang DX, Yan FL (2011) Oxytocin, but not arginine vasopressin is involving in the antinociceptive role of hypothalamic supraoptic nucleus. Peptides 32(5):1042–1046
Acknowledgments
We thank Dr. Maurice Manning for generously providing the oxytocin receptor antagonist. We also thank Rodrigue Maloumby, Andreas Thuy, and Doris Bayerl for the technical assistance. IT received financial support from the Bayerische Forschungsstiftung. IDN received financial support from BMBF and Deutsche Forschungsgemeinschaft.
Disclosure/conflict of interest
The authors report no biomedical financial interest or potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toth, I., Neumann, I.D. & Slattery, D.A. Central administration of oxytocin receptor ligands affects cued fear extinction in rats and mice in a timepoint-dependent manner. Psychopharmacology 223, 149–158 (2012). https://doi.org/10.1007/s00213-012-2702-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-012-2702-4