Abstract
Rationale
Impulsivity is a key feature of disorders that include attention-deficit/hyperactivity disorder (ADHD). The cliff avoidance reaction (CAR) assesses maladaptive impulsive rodent behavior. Dopamine transporter knockout (DAT-KO) mice display features of ADHD and are candidates in which to test other impulsive phenotypes.
Objectives
Impulsivity of DAT-KO mice was assessed in the CAR paradigm. For comparison, attentional deficits were also assessed in prepulse inhibition (PPI) in which DAT-KO mice have been shown to exhibit impaired sensorimotor gating.
Results
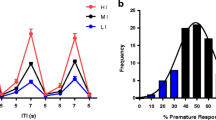
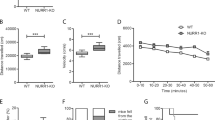
DAT-KO mice exhibited a profound CAR impairment compared to wild-type (WT) mice. As expected, DAT-KO mice showed PPI deficits compared to WT mice. Furthermore, the DAT-KO mice with the most impaired CAR exhibited the most severe PPI deficits. Treatment with methylphenidate or nisoxetine ameliorated CAR impairments in DAT-KO mice.
Conclusion
These results suggest that DAT-KO mice exhibit impulsive CAR behavior that correlates with their PPI deficits. Blockade of monoamine transporters, especially the norepinephrine transporter (NET) in the prefrontal cortex (PFC), may contribute to pharmacological improvement of impulsivity in these mice.






Similar content being viewed by others
References
Arime Y, Kubo Y, Sora I (2011) Animal models of attention-deficit/hyperactivity disorder. Biol Pharm Bull 34(9):1373–1376. doi:10.1248/bpb.34.1373
Arime Y, Kasahara Y, Hall FS, Uhl GR, Sora I (2012) Cortico-subcortical neuromodulation involved in the amelioration of prepulse inhibition deficits in dopamine transporter knockout mice. Neuropsychopharmacology 37(11):2522–2530. doi:10.1038/npp.2012.114
Arnsten AF, Pliszka SR (2011) Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol Biochem Behav 99(2):211–216. doi:10.1016/j.pbb.2011.01.020
Aron AR, Dowson JH, Sahakian BJ, Robbins TW (2003) Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry 54(12):1465–1468. doi:10.1016/S0006-3223(03)00609-7
Barkley RA (1997) Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 121(1):65–94. doi:10.1037/0033-2909.121.1.65
Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelly AE, Schmeichel B, Hamilton C, Spencer RC (2006) Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry 60(10):1111–1120. doi:10.1016/j.biopsych.2006.04.022
Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW (2002) Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 27(5):699–711. doi:10.1016/S0893-133X(02)00346-9
Creese I, Iversen SD (1973) Blockage of amphetamine induced motor stimulation and stereotypy in the adult rat following neonatal treatment with 6-hydroxydopamine. Brain Res 55(2):369–382. doi:10.1016/0006-8993(73)90302-8
Cyr M, Beaulieu JM, Laakso A, Sotnikova TD, Bohn LM, Gainetdinov RR, Caron MG (2003) Sustained elevation of extracellular dopamine causes motor dysfunction and selective degeneration of striatal GABAergic neurons. Proc Natl Acad Sci U S A 100(19):11035–11040. doi:10.1073/pnas.1831768100
Dalley JW, Mar AC, Economidou D, Robbins TW (2008) Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Phamacol Biochem Behav 90(2):250–260. doi:10.1016/j.pbb.2007.12.021
DeVito EE, Balckwell AD, Clark L, Kent L, Dezsery AM, Turner DC, Aitken MR, Sahakian BJ (2009) Methylphenidate improves response inhibition but not reflection-impulsivity in children with attention deficit hyperactivity disorder (ADHD). Psychopharmacol (Berl) 202(1–3):531–539. doi:10.1007/s00213-008-1337-y
Eagle DM, Baunez C (2010) Is there an inhibitory-response-control system in the rat? Evidence from anatomical and pharmacological studies of behavioral inhibition. Neurosci Biobehav Rev 34(1):50–72. doi:10.1016/j.neubiorev.2009.07.003
Engert V, Pruessner JC (2008) Dopaminergic and noradrenergic contributions to functionality in ADHD: the role of methylphenidate. Curr Neuropharmacol 6(4):322–328. doi:10.2174/157015908787386069
Fernagut PO, Chalon S, Diguet E, Guilloteau D, Tison F, Jaber M (2003) Motor behaviour deficits and their histopathological and functional correlates in the nigrostriatal system of dopamine transporter knockout mice. Neuroscience 116(4):1123–1130. doi:10.1016/S0306-4522(02)00778-9
Gainetdinov RR, Westel WC, Jones SR, Levin ED, Jaber M, Caron MG (1999) Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science 285(5400):397–401. doi:10.1126/science.283.5400.397
Goddard AW, Ball SG, Martinez J, Robinson MJ, Yang CR, Russell JM, Shekhar A (2010) Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depress Anxiety 27(4):339–350. doi:10.1002/da.20642
Haenisch B, Bonisch H (2011) Depression and antidepressants: insights from knockout of dopamine, serotonin or noradrenaline re-uptake transporters. Pharmacol Ther 129(3):352–368. doi:10.1016/j.pharmthera.2010.12.002
Hernandez LF, Segovia G, Mora F (2008) Chronic treatment with a dopamine uptake blocker changes dopamine and acetylcholine but not glutamate and GABA concentrations in prefrontal cortex, striatum and nucleus accumbens of the awake rat. Neurochem Int 52(3):457–469. doi:10.1016/j.neuint.2007.08.005
Hiramatsu M, Cho AK, Nabeshima T (1989) Comparison of the behavioral and biochemical effects of the NMDA receptor antagonists, MK-801 and phencyclidine. Eur J Pharmacol 166(3):359–366. doi:10.1016/0014-2999(89)90346-4
Humby T, Wilkinson LS (2011) Assaying dissociable elements of behavioural inhibition and impulsivity: translational utility of animal models. Curr Opin Pharmacol 11(5):534–539. doi:10.1016/j.coph.2011.06.006
Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG (1998) Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci U S A 95(7):4029–4034
Koda K, Ago Y, Cong Y, Kita Y, Takuma K, Matsuda T (2010) Effects of acute and chronic administration of atomoxetine and methylphenidate on extracellular levels of noradrenaline, dopamine and serotonin in the prefrontal cortex and striatum of mice. J Neurochem 114(1):259–270. doi:10.1111/j.1471-4159.2010.06750.x
Kumakura K, Nomura H, Toyoda T, Hashikawa K, Noguchi T, Takeda K, Ichijo H, Tsunoda M, Funatsu T, Ikegami D, Narita M, Suzuki T, Matsuki N (2010) Hyperactivity in novel environment with increased dopamine and impaired novelty preference in apoptosis signal-regulating kinase 1 (ASK1)-deficient mice. Neurosci Res 66(3):313–320. doi:10.1016/j.neures.2009.12.003
Kuroda K, Yamada S, Tanaka M, Iizuka M, Yano H, Mori D, Tsuboi D, Nishioka T, Namba T, Iizuka Y, Kubota S, Nagai T, Ibi D, Wang R, Enomoto A, Isotani-Sakakibara M, Asai N, Kimura K, Kiyonari H, Abe T, Mizoguchi A, Sokabe M, Takahashi M, Yamada K, Kaibuchi K (2011) Behavioral alterations associated with targeted disruption of exons 2 and 3 of the Disc1 gene in the mouse. Hum Mol Genet 20(23):4666–4683. doi:10.1093/hmg/ddr400
Matsuoka Y, Furuyashiki T, Yamada K, Nagai T, Bito H, Tanaka Y, Kitaoka S, Ushikubi F, Nabeshima T, Narumiya S (2005) Prostaglandin E receptor EP1 controls impulsive behavior under stress. Proc Natl Acad Sci U S A 102(44):16066–16071. doi:10.1073/pnas.0504908102
Nigg JT (2000) On inhibition/disinhibition in developmental psychopathology: views from cognitive and personality psychology and a working inhibition taxonomy. Psychol Bull 126(2):220–246. doi:10.1037/0033-2909.126.2.220
Pattij T, Vanderschuren LJ (2008) The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci 29(4):192–199. doi:10.1016/j.tips.2008.01.002
Pogorelov VM, Rodriguiz RM, Insco ML, Caron MG, Westel WC (2005) Novelty seeking and stereotypic activation of behavior in mice with disruption of the Dat1 gene. Neuropsychopharmacology 30(10):1818–1831. doi:10.1038/sj.npp.1300724
Powell SB, Zhou X, Geyer MA (2009) Prepulse inhibition and genetic mouse models of schizophrenia. Behav Brain Res 204(2):282–294. doi:10.1016/j.bbr.2009.04.021
Ralph RJ, Paulus MP, Fumagalli F, Caron MG, Geyer MA (2001) Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: differential effects of D1 and D2 receptor antagonists. J Neurosci 21(1):305–313
Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI (1998) Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex echibit sparse immunoreactivity for the dopamine transporter. J Neurosci 18(7):2697–2708
Shen HW, Hagino Y, Kobayashi H, Shinohara-Tanaka K, Ikeda K, Yamamoto T, Lesch KP, Murphy DL, Hall FS, Uhl GR, Sora I (2004) Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacology 29(10):1790–1799. doi:10.1038/sj.npp.1300476
Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, Lesch KP, Murphy DL, Uhl GR (1998) Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mouse. Proc Natl Acad Sci U S A 95(13):7699–7704
Spielewoy C, Roubert C, Hamon M, Nosten-Bertrand M, Betancur C, Giros B (2000) Behavioural disturbances associated with hyperdopaminergia in dopamine-transporter knockout mice. Behav Pharmacol 11(3–4):279–290
Tripp G, Wickens JR (2009) Neurobiology of ADHD. Neuropharmacology 57(7–8):579–589. doi:10.1016/j.neuropharm.2009.07.026
Uchiumi O, Kasahara Y, Fukui A, Hall FS, Uhl GR, Sora I (2013) Serotonergic involvement in the amelioration of behavioral abnormalities in dopamine transporter knockout mice by nicotine. Neuropharmacology 64:348–356. doi:10.1016/j.neuropharm.2012.07.016
van der Kooij MA, Glennon JC (2007) Animal models concerning the role of dopamine in attention-deficit hyperactivity disorder. Neurosci Biobehav Rev 31(4):597–618. doi:10.1016/j.neubiorev.2006.12.002
Wehmeier PM, Schacht A, Wolff C, Otto WR, Dittmann RW, Banaschewski T (2011) Neuropsychological outcomes across the day in children with attention-deficit/hyperactivity disorder treated with atomoxetine: results from a placebo-controlled study using a computer-based continuous performance test combined with an infra-red motion-tracking device. J Child Adolesc Psychopharmacol 21(5):433–444. doi:10.1089/cap.2010.0142
Winstanley CA (2011) The utility of rat models of impulsivity in developing pharmacotherapies for impulse control disorders. Br J Pharmacol 164(4):1301–1321. doi:10.1111/j.1476-5381.2011.01323.x
Xu TX, Sotnikova TD, Liang C, Zhang J, Jung JU, Spealman RD, Gainetdinov RR, Yao WD (2009) Hyperdopaminergic tone erodes prefrontal long-term potential via a D2 receptor-operated protein phosphatase gate. J Neurosci 29(45):14086–14099. doi:10.1523/JNEUROSCI.0974-09.2009
Yamamoto A, Lucas JJ, Hen R (2000) Reversal of neuropathology and motor dysfunction in a conditional model of Huntington’s disease. Cell 101(1):57–66. doi:10.1016/S0092-8674(00)80623-6
Yamashita M, Fukushima S, Shen HW, Hall FS, Uhl GR, Numachi Y, Kobayashi H, Sora I (2006) Norepinephrine transporter blockade can normalize the prepulse inhibition deficits found in dopamine transporter knockout mice. Neuropsychopharmacology 31(10):2132–2139. doi:10.1038/sj.npp.1301009
Yoshida S, Numachi Y, Matsuoka H, Sato M (1998) Impairment of cliff avoidance reaction induced by subchronic methamphetamine administration and restraint stress: comparison between two inbred strains of rats. Prog Neuropsychopharmacol Biol Psychiatry 22(6):1023–1032. doi:10.1016/S0278-5846(98)00050-5
Young JW, van Enkhuizen J, Winstanley CA, Geyer MA (2011) Increased risk-taking behavior in dopamine transporter knockdown mice: future support for a mouse model of mania. J Psychopharmacol 25(7):934–943. doi:10.1177/0269881111400646
Acknowledgments
This study was supported by a Grant-in-Aid for Health and Labour Science Research (Research on Pharmaceutical and Medical Safety) from MHLW of Japan; by Grants-in-Aid for Core Research for Evolutional Science and Technology (CREST), Global COE Program (Basic & Translational Research Center for Global Brain Science) from MEXT of Japan and through funding from the Intramural Research Program of the National Institute on Drug Abuse, NIH/DHHS, USA (GRU and FSH). All animal experiments were performed in accordance with the guidelines of the Animal Ethics Committee at Tohoku University Graduate School of Medicine (Sendai, Japan). No authors have any other conflicts of interest or financial disclosures to make.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamashita, M., Sakakibara, Y., Hall, F.S. et al. Impaired cliff avoidance reaction in dopamine transporter knockout mice. Psychopharmacology 227, 741–749 (2013). https://doi.org/10.1007/s00213-013-3009-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3009-9