Abstract
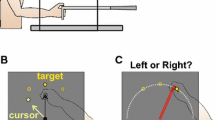
The kinematics of straight reaching movements can be specified vectorially by the direction of the movement and its extent. To explore the representation in the brain of these two properties, psychophysical studies have examined learning of visuomotor transformations of either rotation or gain and their generalization. However, the neuronal substrates of such complex learning are only beginning to be addressed. As an initial step in ensuring the validity of such investigations, it must be shown that monkeys indeed learn and generalize visuomotor transformations in the same manner as humans. Here, we analyze trajectories and velocities of movements as monkeys adapt to either rotational or gain transformations. We used rotations with different signs and magnitudes, and gains with different signs, and analyzed transfer of learning to untrained movements. The results show that monkeys can adapt to both types of transformation with a time course that resembles human learning. Analysis of the aftereffects reveals that rotation is learned locally and generalizes poorly to untrained directions, whereas gain is learned more globally and can be transferred to other amplitudes. The results lend additional support to the hypothesis that reaching movements are learned locally but can be easily rescaled to other magnitudes by scaling the peak velocity. The findings also indicate that reaching movements in monkeys are planned and executed very similarly to those in humans. This validates the underlying presumption that neuronal recordings in primates can help elucidate the mechanisms of motor learning in particular and motor planning in general.








Similar content being viewed by others
References
Abeele S, Bock O (2001) Mechanisms for sensorimotor adaptation to rotated visual input. Exp Brain Res 139:248–253
Ahissar M, Hochstein S (1997) Task difficulty and the specificity of perceptual learning. Nature 387:401–406
Bock O (1992) Adaptation of aimed arm movements to sensorimotor discordance: evidence for direction-independent gain control. Behav Brain Res 51:41–50
Bock O, Burghoff M (1997) Visuo-motor adaptation: evidence for a distributed amplitude control system. Behav Brain Res 89:267–273
Chou IH, Lisberger SG (2002) Spatial generalization of learning in smooth pursuit eye movements: implications for the coordinate frame and sites of learning. J Neurosci 22:4728–4739
Conditt MA, Gandolfo F, Mussa-Ivaldi FA (1997) The motor system does not learn the dynamics of the arm by rote memorization of past experience. J Neurophysiol 78:554–560
Cunningham HA (1989) Aiming error under transformed spatial mappings suggests a structure for visual-motor maps. J Exp Psychol Hum Percept Perform 15:493–506
Donchin O, Francis JT, Shadmehr R (2003) Quantifying generalization from trial-by-trial behavior of adaptive systems that learn with basis functions: theory and experiments in human motor control. J Neurosci 23:9032–9045
Doyon J, Owen AM, Petrides M, Sziklas V, Evans AC (1996) Functional anatomy of visuomotor skill learning in human subjects examined with positron emission tomography. Eur J Neurosci 8:637–648
Georgopoulos AP (1995) Current issues in directional motor control. Trends Neurosci 18:506–510
Georgopoulos AP, Kalaska JF, Caminiti R, Massey JT (1982) On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. J Neurosci 2:1527–1537
Ghahramani Z, Wolpert DM, Jordan MI (1996) Generalization to local remappings of the visuomotor coordinate transformation. J Neurosci 16:7085–7096
Goodbody SJ, Wolpert DM (1998) Temporal and amplitude generalization in motor learning. J Neurophysiol 79:1825–1838
Gordon J, Ghez C (1987) Trajectory control in targeted force impulses. II. Pulse height control. Exp Brain Res 67:241–252
Gordon J, Ghilardi MF, Ghez C (1994) Accuracy of planar reaching movements. I. Independence of direction and extent variability. Exp Brain Res 99:97–111
Gribble PL, Scott SH (2002) Overlap of internal models in motor cortex for mechanical loads during reaching. Nature 417:938–941
Imamizu H, Shimojo S (1995) The locus of visual-motor learning at the task or manipulator level: implications from intermanual transfer. J Exp Psychol Hum Percept Perform 21:719–733
Imamizu H, Uno Y, Kawato M (1995) Internal representations of the motor apparatus: implications from generalization in visuomotor learning. J Exp Psychol Hum Percept Perform 21:1174–1198
Imamizu H, Miyauchi S, Tamada T, et al. (2000) Human cerebellar activity reflecting an acquired internal model of a new tool [see comments]. Nature 403:192–195
Inoue K, Kawashima R, Satoh K, et al. (2000) A PET study of visuomotor learning under optical rotation. Neuroimage 11:505–516
Kalaska JF, Scott SH, Cisek P, Sergio LE (1997) Cortical control of reaching movements. Curr Opin Neurobiol 7:849–859
Kawato M (1999) Internal models for motor control and trajectory planning. Curr Opin Neurobiol 9:718–727
Kitazawa S, Yin PB (2002) Prism adaptation with delayed visual error signals in the monkey. Exp Brain Res 144:258–261
Krakauer JW, Pine ZM, Ghilardi MF, Ghez C (2000) Learning of visuomotor transformations for vectorial planning of reaching trajectories. J Neurosci 20:8916–8924
Krakauer JW, Ghilardi MF, Mentis M, Barnes A, Veytsman M, Eidelberg D, Ghez C (2004) Differential cortical and subcortical activations in learning rotations and gains for reaching: a PET study. J Neurophysiol 91:924–933
Lackner JR, DiZio P (1994) Rapid adaptation to Coriolis force perturbations of arm trajectory. J Neurophysiol 72:299–313
Li CS, Padoa-Schioppa C, Bizzi E (2001) Neuronal correlates of motor performance and motor learning in the primary motor cortex of monkeys adapting to an external force field. Neuron 30:593–607
Ojakangas CL, Ebner TJ (1991) Scaling of the metrics of visually-guided arm movements during motor learning in primates. Exp Brain Res 85:314–323
Paz R, Vaadia E (2004) Learning-induced improvement in encoding and decoding of specific movement directions by neurons in the primary motor cortex. PLoS Biol 2:E45
Paz R, Boraud T, Natan C, Bergman H, Vaadia E (2003) Preparatory activity in motor cortex reflects learning of local visuomotor skills. Nat Neurosci 6:882–890
Pine ZM, Krakauer JW, Gordon J, Ghez C (1996) Learning of scaling factors and reference axes for reaching movements. Neuroreport 7:2357–2361
Sainburg RL, Ghez C, Kalakanis D (1999) Intersegmental dynamics are controlled by sequential anticipatory, error correction, and postural mechanisms. J Neurophysiol 81:1045–1056
Scott SH (2003) The role of primary motor cortex in goal-directed movements: insights from neurophysiological studies on non-human primates. Curr Opin Neurobiol 13:671–677
Shadmehr R, Moussavi ZM (2000) Spatial generalization from learning dynamics of reaching movements. J Neurosci 20:7807–7815
Shadmehr R, Mussa-Ivaldi FA (1994) Adaptive representation of dynamics during learning of a motor task. J Neurosci 14:3208–3224
Toni I, Ramnani N, Josephs O, Ashburner J, Passingham RE (2001) Learning arbitrary visuomotor associations: temporal dynamic of brain activity. Neuroimage 14:1048–1057
Vindras P, Viviani P (2002) Altering the visuomotor gain. Evidence that motor plans deal with vector quantities. Exp Brain Res 147:280–295
Vindras P, Desmurget M, Prablanc C, Viviani P (1998) Pointing errors reflect biases in the perception of the initial hand position. J Neurophysiol 79:3290–3294
Wise SP, Moody SL, Blomstrom KJ, Mitz AR (1998) Changes in motor cortical activity during visuomotor adaptation. Exp Brain Res 121:285–299
Wolpert DM, Ghahramani Z (2000) Computational principles of movement neuroscience. Nat Neurosci 3 Suppl: 1212–1217
Wolpert DM, Ghahramani Z, Jordan MI (1995) An internal model for sensorimotor integration. Science 269:1880–1882
Acknowledgements
This study was supported in part by a Center for Excellence grant (8006/00) administered by the ISF, by BMBF-DIP, by grant 2001073 administered by the BSF, and by a special contribution from the Golden Charitable Trust. R.P. was supported by a Constantiner fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paz, R., Nathan, C., Boraud, T. et al. Acquisition and generalization of visuomotor transformations by nonhuman primates. Exp Brain Res 161, 209–219 (2005). https://doi.org/10.1007/s00221-004-2061-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-004-2061-4