Abstract
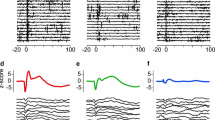
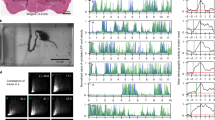
We recorded from over 250 single cortical neurons throughout the medial anterior lobe of the cat cerebellum during passive movements of the ipsilateral hindlimb resembling stepping on a moving treadmill. We applied three different quantitative analysis techniques to determine the extent of neuronal modulation that could be accounted for by the stepping movements. The analyses all indicated that up to half the recorded neurons in all five lobules responded to these passive hindlimb movements. We reconstructed the locations of the recorded neurons on a 2-D map of the cerebellar cortex in order to determine the spatial distribution of responsive cells. Cells that were located in the classical hindlimb projection areas of the anterior lobe (in lobules 2 and 3) were generally most responsive to the limb movement with activity patterns that generally had a linear relationship to hindlimb kinematics. Cells in lobules 4 and 5, considered as classical forelimb areas of the cerebellum, were also responsive. Although these cells tended to have noisier firing patterns, many were found to be modulated nevertheless by the hindlimb movements. We also found a clear demarcation between zones b and c, with a higher fraction of responsive cells in all lobules located in zone b.







Similar content being viewed by others
References
Adrian ED (1943) Afferent areas in the cerebellum connected with the limbs. Brain 66:289–315
Apps R, Lidierth M (1989) Simple spike discharge patterns of Purkinje cells in the paramedian lobule ofthe cerebellum during locomotion in the awake cat. Neurosci Lett 102(2–3):205–210
Armstrong DM, Edgley SA (1984) Discharges of Purkinje cells in the paravermal part of the cerebellar anterior lobe during locomotion in the cat. J Physiol 352:403–424
Bosco G, Poppele RE (2002) Encoding of hindlimb kinematics by spinocerebellar circuitry. Arch Ital Biol 140:185–192
Bosco G, Rankin A, Poppele RE (1996) A representation of passive hindlimb postures in cat spinocerebellar activity. J Neurophysiol 76:715–726
Bosco G, Poppele RE, Eian J (2000) Reference frames for spinal proprioception: limb endpoint based or joint-level based? J Neurophysiol 83(5):2931–2945
Bosco G, Eian J, Poppele RE (2005) Kinematic and non-kinematic signals transmitted to the cat cerebellum during passive treadmill stepping. Exp Brain Res 167(3):394–403
Bosco G, Eian J, Poppele RE (2006) Phase-specific sensory representations in spinocerebellar activity during stepping: evidence for a hybrid kinematic/kinetic framework. Exp Brain Res 175(1):83–96
Bower JM (2002) The organization of cerebellar cortical circuitry revisited: implications for function. Ann NY Acad Sci 978:135–155
Bower JM, Woolston DC (1983) Congruence of spatial organization of tactile projections to granule cell and Purkinje cell layers of cerebellar hemispheres of the albino rat: vertical organization of cerebellar cortex. J Neurophysiol 49:745–766
Cohen D, Yarom Y (1998) Patches of synchronized activity in the cerebellar cortex evoked by mossy-fiber stimulation: questioning the role of parallel fibers. Proc Natl Acad Sci USA 95(25):15032–15036
Edgley SA, Lidierth M (1988) Step-related discharges of Purkinje cells in the paravermal cortex of the cerebellar anterior lobe in the cat. J Physiol 401:399–415
Ekerot CF, Jorntell H (2001) Parallel fibre receptive fields of Purkinje cells and interneurons are climbing fibre-specific. Eur J Neurosci 13(7):1303–1310
Ekerot CF, Jorntell H (2003) Parallel fiber receptive fields: a key to understanding cerebellar operation and learning. Cerebellum 2(2):101–109
Garwicz M, Jorntell H, Ekerot CF (1998) Cutaneous receptive fields and topography of mossy fibres and climbing fibres projecting to cat cerebellar C3 zone. J Physiol 512(Pt 1):277–293
Gundappa-Sulur G, De Schutter E, Bower JM (1999) Ascending granule cell axon: an important component of cerebellar cortical circuitry. J Comp Neurol 408(4):580–596
Holmes G. (1939) The cerebellum of man. Brain 62:1–30
Holtzman T, Rajapaksa T, Mostofi A, Edgley SA (2006) Different responses of rat cerebellar Purkinje cells and Golgi cells evoked by widespread convergent sensory inputs. J Physiol 574(Pt 2):491–507
Lu H, Hartmann MJ, Bower JM (2005) Correlations between purkinje cell single-unit activity and simultaneously recorded field potentials in the immediately underlying granule cell layer. J Neurophysiol 94(3):1849–1860
MacKay WA, Murphy JT (1976) Integrative versus delay line characteristics of cerebellar cortex. Can J Neurol Sci 3(2):85–97
Pardoe J, Edgley SA, Drew T, Apps R (2004) Changes in excitability of ascending and descending inputs to cerebellar climbing fibers during locomotion. J Neurosci 24(11):2656–2666
Pijpers A, Apps R, Pardoe J, Voogd J, Ruigrok TJ (2006) Precise spatial relationships between mossy fibers and climbing fibers in rat cerebellar cortical zones. J Neurosci 26(46):12067–12080
Perciavalle V, Bosco G, Poppele R (1995) Correlated activity in the spinocerebellum is related to spinal timing generators. Brain Res 695:293–297
Perciavalle V, Bosco G, Poppele R (1998) Spatial organization of proprioception in the cat spinocerebellum. Purkinje cell responses to passive foot rotation. Eur J Neurosci 10(6):1975–1985
Poppele RE, Bosco G, Rankin AM (2002) Independent representations of limb axis length and orientation in spinocerebellar response components. J Neurophysiol 87(1):409–422
Poppele RE, Rankin A, Eian J (2003) Dorsal spinocerebellar tract neurons respond to contralateral limb stepping. Exp Brain Res 149(3):361–370
Rubia FJ, Tandler R (1981) Spatial distribution of afferent information to the anterior lobe of the cat’s cerebellum. Exp Brain Res 42:249–259
Santamaria F, Tripp PG, Bower JM (2007) Feedforward inhibition controls the spread of granule cell-induced Purkinje cell activity in the cerebellar cortex. J Neurophysiol 97(1):248–263
Shambes GM, Gibson JM, Welker W (1978) Fractured somatotopy in granule cell tactile areas of rat cerebellar hemispheres revealed by micromapping. Brain Behav Evol 15(2):94–140
Sillitoe RV, Hulliger M, Dyck R, Hawkes R (2003) Antigenic compartmentation of the cat cerebellar cortex. Brain Res 977:1–15
Snider RS, Stowell A (1944) Receiving areas of the tactile, auditory and visual systems in the cerebellum. J Neurophisiol 7:331–357
Thach WT, (1967) Somatosensory receptive fields of single units in cat cerebellar cortex. J Neurophysiol 30(4):675–696
Thach WT, Bastian AJ (2004) Role of the cerebellum in the control and adaptation of gait in health and disease. Prog Brain Res 143:353–366 (Review)
Valle MS, Bosco G, Poppele RE (2000) Information processing in the spinocerebellar system. Neuroreport 11(18):4075–4079
Welker W (1987) Spatial organization of somatotosensory projections to granule cell cerebellar cortex: functional connectional implications of fractured somatotopapy (summary of Wisconsin studies). In: King JS (ed) New concepts in cerebellar neurobiology. Alan Liss, New York, pp 239–280
Wilkinson L (1990) The system for statistic. SYSTAT, Evanston
Acknowledgments
This research was supported by a grant from the USPHS, NIH grant R01 NS21143. The authors thank Dr. T, Ebner for helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valle, M.S., Eian, J., Bosco, G. et al. Cerebellar cortical activity in the cat anterior lobe during hindlimb stepping. Exp Brain Res 187, 359–372 (2008). https://doi.org/10.1007/s00221-008-1311-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-008-1311-2