Abstract
Background: Magnetization transfer contrast and magnetization transfer ratio (MTR) in brain are mainly related to the presence of myelin. Neuropathological studies of brain lesions in tuberous sclerosis complex (TSC) have demonstrated disordered myelin sheaths. Objective: To evaluate the MTR of the brain in children with TSC and to compare with that in controls. Materials and methods: Four patients (aged 0.41–8.4 years, mean 2.5 years) with TSC and four age- and sex-matched controls were evaluated with classic MR sequences and with a three-dimensional gradient-echo sequence without and with magnetization transfer pre-pulse. The MTR was calculated as: (SI0−SIm)/SI0×100%, where SIm refers to signal intensity from an image acquired with a magnetization transfer pre-pulse and SI0 the signal intensity from the image acquired without a magnetization transfer pre-pulse. Results: The MTR values of cortical tubers (44.1±4.1), of subependymal nodules (51.6±4.8) and of white matter lesions (52.4±1.8) were significantly lower than those of cortex (58.7±3.53), of basal ganglia (caudate nucleus 58.2±2.8, putamen 59.6±2.5, thalamus 61.3±2.4) and of white matter (64.2±2.5) in controls (P<0.001). The MTR of normal-appearing white matter (61.2±3.0) in patients was lower than that of white matter in controls (P<0.01). The MTR of cortex and basal ganglia in patients was not significantly different from that in controls. Conclusions: MTR measurements not only provide semiquantitative information for TSC lesions but also reveal more extensive disease.
Similar content being viewed by others
Introduction
Tuberous sclerosis complex (TSC) is a phakomatosis characterized by the presence of hamartomatous lesions in the brain, eyes, kidneys, heart and skin [1, 2]. It results from mutations in two tumour suppressor genes, TSC1 (on chromosome 9q34) encoding a protein called hamartin, and TSC2 (on chromosome 16p13.3) encoding a protein called tuberin [1–3]. TSC may be transmitted as an autosomal dominant trait with variable penetrance, but in 65% of cases its occurrence is sporadic [1, 2]. Its prevalence is approximately 1 in 10,000 live births, but it may be underestimated because of paucisymptomatic forms [3].
Imaging manifestations of brain lesions are of considerable importance, and this is emphasized in the revised diagnostic criteria for TSC [4]. CNS lesions (cortical tubers, radial white-matter anomalies, subependymal nodules and subependymal giant-cell astrocytoma and cyst-like lesions) have been mainly studied with classic MR sequences [5, 6]. However, there is an increasing interest in other MR imaging techniques that might provide hints as to the real extension of brain disease. Diffusion techniques, by detecting changes in random motion of water molecules, provide tissue information about microscopic barriers which are cellular membranes [7]. Diffusion imaging has been used in some studies of TSC and the apparent diffusion coefficient (ADC) of lesions and normal-appearing white matter (NAWM) has been measured [8–10]. Magnetization transfer (MT) techniques enable semiquantitative tissue characterization (magnetization transfer ratio, MTR) using the phenomenon of saturation transfer between immobile macromolecular protons and the mobile water protons [11, 12]. Macromolecular protons are found in proteins and cellular membranes. MTR is determined by the field strength and the scanning parameters, but principally by the concentration of macromolecules and the efficacy of interaction between the bound and free pool of protons [11, 12]. There is only one study in which MT techniques were used to evaluate the MTR of brain in TSC [13]. In this study, measurements were obtained using a spin-echo T1-weighted (T1-W) sequence which, in contrast to proton density-weighted sequences, have the disadvantage of underestimating the MTR by approximately 50% [14, 15].
The purpose of this study was to evaluate in patients with TSC the MTR of different types of brain lesions, of NAWM and of grey matter using three-dimensional gradient-echo proton density-weighted sequence before and after the application of a MT pre-pulse.
Materials and methods
Brain MRI examinations of four children (three girls, one boy; age range 0.41–8.4 years, mean 2.5 years) with TSC according to clinical diagnostic criteria [4] were retrospectively evaluated and compared with those of four age- and sex-matched controls. Control subjects were evaluated for either headache or suspicion of meningoencephalitis and had a normal brain MRI.
All MRI examinations were performed on the same 1.5-T MR unit (Gyroscan ACS NT; Philips Medical Systems, Best, The Netherlands) using a head coil, a field of view of 24 cm and a 256×256 acquisition matrix. Sequences were: axial T2-W turbo spin-echo (TR/TE 3500/90; slice thickness 5 mm; intersection gap 0.5 mm), coronal T2-W turbo spin-echo (TR/TE 3500/90; slice thickness 2.5 mm; intersection gap 0 mm), sagittal unenhanced T1-W spin-echo (TR/TE 650/17; slice thickness 4 mm, intersection gap 0.4 mm) and contrast-enhanced axial, coronal and sagittal T1-W spin-echo.
To study the MT phenomenon a three-dimensional gradient-echo sequence (TR/TE 32/8, 6° flip angle) was performed and 4 mm/2 mm overlapping sections were concurrently obtained without and with the application of a MT binomial pre-pulse (1–2–1) applied on-resonance. Images of this sequence obtained before the application of the MT pre-pulse had proton-density contrast due to the short TE and the small flip angle (Fig. 1). The MT sequence performed in the axial plane (parallel to the intercommisural line, i.e. the anterior commissure to posterior commissure line or AC–PC line) and coronal plane (perpendicular to the AC–PC line) were part of our standard protocol. Images obtained after the application of the MT pre-pulse depict structures of high MTR with low signal intensity and those of low MTR with higher intensity (Fig. 1). The duration of a complete MR examination ranged from 15 to 25 min. All MR examinations were evaluated by a paediatric neuroradiologist (M.I.A.) who was aware of each subject’s clinical condition. Brain lesions were evaluated on T2-W unenhanced and contrast-enhanced T1-W images. Based on conventional MR findings, the signal intensity of the subependymal nodules, white-matter lesions, cortical tubers, NAWM, cortex, putamina, caudate nuclei and thalami were evaluated using the region-of-interest (ROI) method. Signal intensity measurements were also performed in the control group in the cortex, putamina, caudate nuclei, thalami and frontal and occipital white matter. The ROIs were circular with a minimum size of 0.5 mm2. The MTR was calculated as:
(SI0−SIm)/SI0×100%
where SIm refers to the signal intensity from an image acquired with a MT pre-pulse and SI0 the signal intensity from the image acquired without a MT pre-pulse.
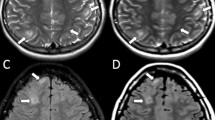
A 17-month-old boy. a Axial proton-density MRI before the application of the MT pre-pulse shows subependymal nodules with intermediate signal intensity (arrows). b After the application of the MT pre-pulse the subependymal nodules (arrows) are of low signal intensity (high MTR) while the CSF has high signal intensity (very low MTR)
Statistical analysis
Statistical analysis was performed using SPSS base 11.5 for Windows. The normality of the distribution of the parameters was assessed by the Kolmogorov–Smirnov test. The unpaired two-tailed Student’s t-test was used to study differences between patients and controls. P values less than 0.05 were considered statistically significant.
Results
Three types of lesions were identified in our series: cortical tubers, subependymal nodules and white-matter lesions. The MTR values of all types of lesions and of the NAWM were significantly lower than the MTR of white matter in controls (Table 1). There were no significant differences in the MTR values of the cortex and the basal ganglia structures between patients and controls (Table 2). The MTR values of cortical tubers (44.1±4.1), white matter lesions (52.4±1.8) and subependymal nodules (51.6±4.8) were significantly lower than the MTR values of the cortex (56.7±2.6) and basal ganglia structures (caudate nucleus 58.0±1.1, putamen 58.7±1.0, thalamus 60.2±1.4; P<0.01). Cortical tubers had a significantly lower MTR than white-matter lesions and subependymal nodules (P<0.001). The MTR of the cortex surrounding the cortical tubers was within the normal range. No significant difference of MTR was found between subependymal nodules and white-matter lesions.
Discussion
According to current concepts, CNS lesions in TSC are the result of disordered cell differentiation, proliferation and migration [1, 3]. Apart from generating neuroblasts, which differentiate into normal neurons and astrocytes, the germinal matrix in TSC gives rise to a second population of primitive giant cells the “neuroastrocytes”, which are deprived of clear neuronal or glial differentiation. Non-migrating neuroastrocytes form the subependymal nodules and the subependymal giant cell astrocytomas, the partially migrating ones form radial white-matter lesions and those migrating at the cortical plate form cortical tubers. Although both cortical tubers and subependymal nodules contain neuroastrocytes, they are histologically distinct lesions [1, 16]. Cortical tubers are areas of dysplastic cortex which have lost their hexalaminar structure and contain neuroastrocytes, neurons and astrocytes [1, 16, 17]. As opposed to cortical tubers, subependymal nodules contain epithelioid and spindle cell populations and show a greater cellular packing density similar to that of tumours [1, 16, 17].
In the present study the MTR values of all detected lesions and that of NAWM were lower than the MTR values of the white matter in controls. There is evidence that the MT contrast in the brain is mainly related to the presence of myelin since experimental studies have demonstrated that the MT effect for myelin water is much larger than that for intra/intercellular water [18]. Neuropathological studies have not only demonstrated depleted and disordered myelin sheaths in TSC lesions, but have also shown at the microscopic level foci of white matter hypomyelination that are not depicted with classic MR imaging [17, 19, 20]. Hypomyelination which, in diffusion studies, seems to be the cause of low ADC values of cortical tubers and of NAWM [8–10], may also explain the low MTR we found in TSC lesions and in NAWM. Another possible underlying cause for the low MTR is microscopic cystic degeneration of TSC lesions [6]. Cystic foci of the lesions deprived of macromolecules may contribute to a decrease of MTR.
Among lesions visualized with classic MR sequences, the highest MTR was present in subependymal nodules. Their high cellular packing density may be responsible for a high membrane density. Membranes contain macromolecular protons that affect MT contrast [11, 12]. Therefore, increased concentration of macromolecular protons may explain the higher MTR of subependymal nodules.
Although cortical tubers had the lowest MTR, that of the neighbouring cortex was within the normal range. This is in agreement with the results of histological studies demonstrating normal cytoarchitecture of the cortex surrounding cortical tubers [1].
Apart from subependymal nodules extending to the caudate nucleus, there is no evidence of macro- or microscopic lesions in the basal ganglia of patients with TSC [17, 21]. This probably explains the normal MTR values of the basal ganglia in our patients.
In a previous study using a spin-echo T1-W sequence the MTR values of brain lesions were evaluated in a series of patients with TSC [13]. In this study the MTR of cortical tubers and of white-matter lesions was not significantly different from the MTR of normal grey matter. Moreover the MTR values of the different types of lesions were much lower than those found in the present study. MT techniques use a pre-pulse which preferentially saturates macromolecular protons. However, macromolecular protons, by transferring magnetization to free water protons, affect their longitudinal magnetization, which decreases exponentially with a new lower time constant (T1sat) [11, 14, 15]. This simultaneous reduction of T1 and of longitudinal magnetization, has an opposite effect on tissue signal, and T1-W sequences could result in an underestimation of MTR by 50% [14, 15]. This could be alleviated by using proton-density weighting and for this reason for MTR measurements we used a sequence with parameters favouring a proton-density contrast.
In conclusion, MT techniques in TSC reveal more extensive disease than classic MR sequences. MTR values provide semiquantitative information useful in the diagnostic work up of these patients.
References
Crino PB, Henske EP (1999) New developments in the neurobiology of the tuberous sclerosis complex. Neurology 53:1384–1390
Narayanan V (2003) Tuberous sclerosis complex: genetics to pathogenesis. Pediatr Neurol 29:404–409
Curatolo P, Verdecchia M, Bombardieri R (2003) Tuberous sclerosis complex: a review of neurological aspects. Eur J Paediatr Neurol 6:15–23
Roach ES, Gomez MR, Northrup H (1998) Tuberous sclerosis complex consensus conference: revised clinical diagnostic criteria. J Child Neurol 13:624–628
Baron Y, Barkovich AJ (1999) MR imaging of tuberous sclerosis in neonates and young infants. AJNR Am J Neuroradiol 20:907–916
Rott HD, Lemcke B, Zenker M, et al (2002) Cyst-like cerebral lesions in tuberous sclerosis. Am J Med Genet 111:435–439
Le Bihan D, Mangin JF, Poupon C, et al (2001) Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 13:534–546
Garaci FG, Floris R, Bozzao A, et al (2004) Increased brain apparent diffusion coefficient in tuberous sclerosis. Radiology 232:461–465
Jansen FE, Braun KP, van Nieuwenhuizen O, et al (2003) Diffusion-weighted magnetic resonance imaging and identification of the epileptogenic tuber in patients with tuberous sclerosis. Arch Neurol 60:1580–1584
Peng SS, Lee WT, Wang YH, et al (2004) Cerebral diffusion tensor images in children with tuberous sclerosis: a preliminary report. Pediatr Radiol 34:387–392
Wolff SD, Balaban RS (1994) Magnetization transfer imaging: practical aspects and clinical applications. Radiology 192:593–599
Kurki T, Lundbom N, Komu M, et al (1996) Tissue characterization of intracranial tumors by magnetization transfer and spin-lattice relaxation parameters in vivo. J Magn Reson Imaging 6:573–579
Jeong MG, Chung TS, Coe CJ, et al (1997) Application of magnetization transfer imaging for intracranial lesions of tuberous sclerosis. J Comput Assist Tomogr 21:8–14
Finelli DA, Reed DR (1998) Flip angle dependence of experimentally determined T1sat and apparent magnetization transfer rate constants. J Magn Reson Imaging 8:548–553
Argyropoulou MI, Kiortsis DN, Metafratzi Z, et al (2001) Magnetisation transfer imaging of the normal adenohypophysis: the effect of sex and age. Neuroradiology 43:305–308
Mizuguchi M, Takashima S (2001) Neuropathology of tuberous sclerosis. Brain Dev 23:508–515
Ridler K, Bullmore ET, De Vries PJ, et al (2001) Widespread anatomical abnormalities of grey and white matter structure in tuberous sclerosis. Psychol Med 31:1437–1446
Stanisz GJ, Kecojevic A, Bronskill MJ, et al (1999) Characterizing white matter with magnetization transfer and T(2). Magn Reson Med 42:1128–1136
Nixon JR, Miller GM, Okazaki H, et al (1989) Cerebral tuberous sclerosis: postmortem magnetic resonance imaging and pathologic anatomy. Mayo Clin Proc 64:305–311
Yagishita A, Arai N (1999) Cortical tubers without other stigmata of tuberous sclerosis: imaging and pathological findings. Neuroradiology 41:428–432
Roske B, Stoltenburg G, Baier PM, et al (2003) Tuberous sclerosis complex with disseminated telencephalic distribution of atypical cells and their relation to corticogenesis. Clin Neuropathol 22:119–128
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00247-005-1588-2
Rights and permissions
About this article
Cite this article
Zikou, A., Ioannidou, MC., Tzoufi, M. et al. Magnetization transfer ratio measurements of the brain in children with tuberous sclerosis complex. Pediatr Radiol 35, 1071–1074 (2005). https://doi.org/10.1007/s00247-005-1539-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-005-1539-y