Abstract
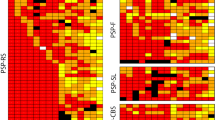
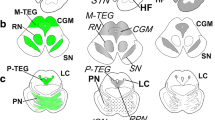
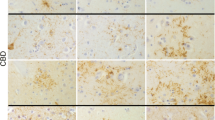
Tufted astrocytes (TAs) are considered reliable, specific markers for the neuropathologic diagnosis of progressive supranuclear palsy (PSP). It is known that neurofibrillary tangles (NFTs) may relate directly to neurodegeneration, but the role of glial tau pathology is not well determined. To examine the hypothesis that TAs are as pathogenic as NFTs and that both might have a common accumulation, we evaluated the topographic relationship between TAs and NFTs in 12 cases of PSP. The sections of 13 different parts of the brain were stained using the Gallyas–Braak method, and TAs and NFTs were counted and compared statistically. The number of TAs significantly correlated with that of NFTs in the central gray matter, pontine nuclei, and tegmentum, which are responsible for the main symptoms in PSP. In the examined allocortex, however, NFTs were abundant without accompanying TAs. Staining with the specific antibody for 4-repeat tau (RD4) and 3-repeat tau (RD3) was performed to clarify this discrepancy from the standpoint of tau isoforms. NFTs in the entorhinal cortex were stained with both RD3 and RD4, but NFTs in the premotor cortex were stained with only RD4. The nature of NFTs in the allocortical area was different from that of the isocortex in PSP. TAs in the isocortex may share the same pathologic cascade with NFTs stained only by RD4. These results suggest that TAs are part of the same pathologic process as NFTs in PSP.





Similar content being viewed by others
References
Akiyama H (2006) Abeta, tau and alpha-synuclein and glial cells. (Article in Japanese) Nihon Shinkei Seishin Yakurigaku Zasshi 26:23–31
Agid Y, Javory-Agid F, Ruberg M, Pillon B, Dubois B, Duyckaerts C, Hauw JJ, Baron JC, Scatton B (1987) Progressive supranuclear palsy: anatomoclinical and biochemical considerations. Adv Neurol 45:191–216
Arai T, Ikeda K, Akiyama H, Nonaka T, Hasegawa M, Ishiguro K, Iritani S, Tsuchiya K, Iseki E, Yagishita S, Oda T, Mochizuki A (2004) Identification of amino-terminally cleaved tau fragments that distinguish progressive supranuclear palsy from corticobasal degeneration. Ann Neurol 55:72–79
Armstrong RA, Lantos PL, Cairns NJ (2007) Progressive supranuclear palsy (PSP): a quantitative study of the pathological changes in cortical and subcortical regions of eight cases. J Neural Transm 114:1569–1577
Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M (2006) Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442:916–919
Bierer LM, Hof PR, Purohit DP, Carlin L, Schmeidler J, Davis KL, Perl DP (1995) Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer’s disease. Arch Neurol 52:81–88
Bigio EH, Brown DF, White CL 3rd (1999) Progressive supranuclear palsy with dementia: cortical pathology. J Neuropathol Exp Neurol 58:359–364
Bouras C, Hof PR, Morrison JH (1993) Neurofibrillary tangle densities in the hippocampal formation in a non-demented population define subgroups of patients with differential early pathologic changes. Neurosci Lett 153:131–135
Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112:389–404
Braak H, Sastre M, Del Tredici K (2007) Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol 114:231–241
Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL 3rd, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DM Consortium for Frontotemporal Lobar Degeneration (2007) Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 114:5–22
Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, Rademakers R, Vandenberghe R, Dermaut B, Martin JJ, van Duijn C, Peeters K, Sciot R, Santens P, De Pooter T, Mattheijssens M, Van den Broeck M, Cuijt I, Vennekens K, De Deyn PP, Kumar-Singh S, Van Broeckhoven C (2006) Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 442:920–924
Delacourte A, David JP, Sergeant N, Buée L, Wattez A, Vermersch P, Ghozali F, Fallet-Bianco C, Pasquier F, Lebert F, Petit H, Di Menza C (1999) The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer’s disease. Neurology 52:1158–1165
de Silva R, Lashley T, Gibb G, Hanger D, Hope A, Reid A, Bandopadhyay R, Utton M, Strand C, Jowett T, Khan N, Anderton B, Wood N, Holton J, Revesz T, Lees A (2003) Pathological inclusion bodies in tauopathies contain distinct complements of tau with three or four microtubule-binding repeat domains as demonstrated by new specific monoclonal antibodies. Neuropathol Appl Neurobiol 29:288–302
Dickson DW (1999) Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol 246(suppl 2):II6–II15
Dickson DW, Rademakers R, Hutton ML (2007) Progressive supranuclear palsy: pathology and genetics. Brain Pathol 17:74–82
Forman MS, Lal D, Zhang B, Dabir DV, Swanson E, Lee VM, Trojanowski JQ (2005) Transgenic mouse model of tau pathology in astrocytes leading to nervous system degeneration. J Neurosci 25:3539–3550
Fukutani Y, Cairns NJ, Shiozawa M, Sasaki K, Sudo S, Isaki K, Lantos PL (2000) Neuronal loss and neurofibrillary degeneration in the hippocampal cortex in late-onset sporadic Alzheimer’s disease. Psychiatry Clin Neurosci 54:523–529
Gibb WR, Luthert PJ, Marsden CD (1989) Corticobasal degeneration. Brain 112:1171–1192
Hanihara T, Amano N, Takahashi H, Nagatomo H, Yagishita S (1995) Distribution of tangles and threads in the cerebral cortex in progressive supranuclear palsy. Neuropathol Appl Neurobiol 21:319–326
Hattori M, Hashizume Y, Yoshida M, Iwasaki Y, Hishiikawa N, Ueda R, Ojika K (2003) Distribution of astrocytic plaques in the corticobasal degeneration brain and comparison with tuft-shaped astrocytes in the progressive supranuclear palsy brain. Acta Neuropathol (Berl) 106:143–149
Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, McKee A, Tabaton M, Litvan I (1994) Preliminary NINDS neuropathological criteria for Steele–Richardson–Olszewski syndrome (progressive supranuclear palsy). Neurology 44:2015–2019
Hof PR, Delacourte A, Bouras C (1992) Distribution of cortical neurofibrillary tangles in progressive supranuclear palsy: a quantitative analysis of six cases. Acta Neuropathol (Berl) 84:45–51
Ikeda K (1996) Glial cytoskeletal abnormalities in neurodegenerative diseases. (1996) No To Shinkei 48:885–894
Iwasaki Y, Yoshida M, Hattori M, Goto A, Aiba I, Hashizume Y, Sobue G (2004) Distribution of tuft-shaped astrocytes in the cerebral cortex in progressive supranuclear palsy. Acta Neuropathol (Berl) 108:399–405
Jin C, Katayama S, Hiji M, Watanabe C, Noda K, Nakamura S, Matsumoto M (2006) Relationship between neuronal loss and tangle formation in neurons and oligodendroglia in progressive supranuclear palsy. Neuropathology 26:50–56
Komori T, Arai N, Oda M, Nakayama H, Mori H, Yagishita S Takahashi T, Amano N, Murayama S, Murakami S, Shibata N, Kobayashi M, Sasaki S, Iwata M (1998) Astrocytic plaques and tufts of abnormal fibers do not coexist in corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol (Berl) 96:401–408
Litvan I, Mangone CA, McKee A, Verny M, Parsa A, Jellinger K, D’Olhaberriague L, Chaudhuri KR, Pearce RK (1996) Natural history of progressive supranuclear palsy (Steele–Richardson–Olszewski syndrome) and clinical predictors of survival: a clinicopathological study. J Neurol Neurosurg Psychiatr 60:615–620
Matusaka H, Ikeda K, Akiyama H, Arai T, Inoue M, Yagishita S (1998) Astrocytic pathology in progressive supranuclear palsy: significance for neuropathological diagnosis. Acta Neuropathol (Berl) 96:248–252
Schmidt ML, Huang R, Martin JA, Henley J, Mawal-Dewan M, Hurtig HI, Lee VM, Trojanowski JQ (1996) Neurofibrillary tangles in progressive supranuclear palsy contain the same tau epitopes identified in Alzheimer’s disease PHFtau. J Neuropathol Exp Neurol 55:534–539
Steele JC, Richardson JC, Olszewski J (1964) Progressive supranuclear palsy. A heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol 10:333–359
Takahashi M, Weidenheim KM, Dickson DW, Ksiezak-Reding H (2002) Morphological and biochemical correlations of abnormal tau filaments in progressive supranuclear palsy. J Neuropathol Exp Neurol 61:33–45
Togo T, Dickson DW (2002) Tau accumulation in astrocytes in progressive supranuclear palsy is a degenerative rather than a reactive process. Acta Neuropathol (Berl) 104:398–402
Troost BT (1995) Neuro-ophthalmological aspects. In: Progressive supranuclear palsy: clinical and research approaches, Oxford University Press, New York, pp184–203
Vermersch P, Frigard B, David JP, Fallet-Bianco C, Delacourte A (1992) Presence of abnormally phosphorylated Tau proteins in the entorhinal cortex of aged non-demented subjects. Neurosci Lett 144:143–146
Vermersch P, Robitaille Y, Bernier L, Wattez A, Gauvreau D, Delacourte A (1994) Biochemical mapping of neurofibrillary degeneration in a case of progressive supranuclear palsy: evidence for general cortical involvement. Acta Neuropathol (Berl) 87:572–577
Wakabayashi K, Oyanagi K, Makifuchi T, Ikuta F, Homma A, Homma Y, Horikawa Y, Tokiguchi S (1994) Corticobasal degeneration: etiopathological significance of the cytoskeletal alterations. Acta Neuropathol 87:545–553
Yamada T, McGeer PL (1990) Oligodendroglial microtubular masses: an abnormality observed in some human neurodegenerative diseases. Neurosci Lett 120:163–166
Yamada T, McGeer PL, McGeer EG (1992) Appearance of paired nucleated, Tau-positive glia in patients with progressive supranuclear palsy brain tissue. Neurosci Lett 135:99–102
Acknowledgments
The technical assistance of both Mrs. Junko Taniguchi and Yasuyo Hano is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ito, K., Arai, K., Yoshiyama, Y. et al. Astrocytic tau pathology positively correlates with neurofibrillary tangle density in progressive supranuclear palsy. Acta Neuropathol 115, 623–628 (2008). https://doi.org/10.1007/s00401-008-0378-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-008-0378-y