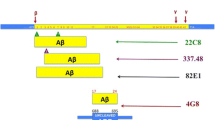
Abstract
To reveal the underlying mechanisms responsible for the regional vulnerability to amyloid-β (Aβ) accumulation prior to the development of Alzheimer’s disease, we studied distribution of Aβ, apolipoprotein E (apoE), synaptic markers, and other molecules involved in Aβ metabolism in multiple brain areas of non-demented individuals. Twelve brain regions including neocortical, limbic, and subcortical areas were dissected from brains of non-demented individuals and extracted according to increasing insolubility by a sequential three-step method. The levels of Aβ40, Aβ42, apoE, APP, APP-CTFβ, BACE1, presenilin-1, neprilysin, insulysin, LRP1, LDLR, synaptophysin, PSD95, GFAP, and lactate were determined by ELISAs or enzymatic assays. The regional distribution of apoE showed moderate-to-strong inverse correlation with levels of Aβ, especially insoluble Aβ40. On the other hand, the regional distributions of synaptic markers, particularly PSD95, showed moderate-to-strong positive correlation with levels of Aβ, especially soluble Aβ40. The regional correlations between Aβ and LRP1, GFAP, or lactate were mild-to-moderate. Moderate-to-strong positive regional correlations were observed between apoE and GFAP or lactate and between PSD95 and LRP1. No significant regional correlations were detected between Aβ and APP, APP-CTFβ, BACE1, or presenilin-1, those involved in Aβ production. There were no significant negative regional correlations between Aβ and two major Aβ degrading enzymes, neprilysin and insulysin. These regional correlations remained consistent regardless of the degree of Aβ accumulation. The regional vulnerability to Aβ accumulation may be due to a net balance between two competing processes: (1) synapses involved in promoting the initial Aβ accumulation and (2) astrocyte-derived apoE involved in preventing Aβ accumulation.





Similar content being viewed by others
References
Akiyama H, Kondo H, Ikeda K, Kato M, McGeer PL (2001) Immunohistochemical localization of neprilysin in the human cerebral cortex: inverse association with vulnerability to amyloid beta-protein (Abeta) deposition. Brain Res 902:277–281
Arold S, Sullivan P, Bilousova T et al (2012) Apolipoprotein E level and cholesterol are associated with reduced synaptic amyloid beta in Alzheimer’s disease and apoE TR mouse cortex. Acta Neuropathol 123:39–52
Bales KR, Liu F, Wu S et al (2009) Human APOE isoform-dependent effects on brain beta-amyloid levels in PDAPP transgenic mice. J Neurosci 29:6771–6779. doi:10.1523/jneurosci.0887-09.2009
Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E (2011) Alzheimer’s disease. Lancet 377:1019–1031. doi:10.1016/s0140-6736(10)61349-9
Benkovic SA, McGowan EM, Rothwell NJ, Hutton M, Morgan DG, Gordon MN (1997) Regional and cellular localization of presenilin-2 RNA in rat and human brain. Exp Neurol 145:555–564. doi:10.1006/exnr.1997.6487
Bernstein HG, Ansorge S, Riederer P, Reiser M, Frolich L, Bogerts B (1999) Insulin-degrading enzyme in the Alzheimer’s disease brain: prominent localization in neurons and senile plaques. Neurosci Lett 263:161–164
Bero AW, Yan P, Roh JH et al (2011) Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci 14:750–756. doi:10.1038/nn.2801
Braak H, Braak E (1991) Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 82:239–259
Bramanti V, Tomassoni D, Avitabile M, Amenta F, Avola R (2010) Biomarkers of glial cell proliferation and differentiation in culture. Front Biosci 2:558–570
Bu G (2009) Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat Rev Neurosci 10:333–344. doi:10.1038/nrn2620
Buckner RL, Snyder AZ, Shannon BJ et al (2005) Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 25:7709–7717. doi:10.1523/jneurosci.2177-05.2005
Camus MC, Chapman MJ, Forgez P, Laplaud PM (1983) Distribution and characterization of the serum lipoproteins and apoproteins in the mouse, Mus musculus. J Lipid Res 24:1210–1228
Chakrabarty P, Jansen-West K, Beccard A et al (2010) Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition. FASEB J 24:548–559. doi:10.1096/fj.09-141754
Cirrito JR, Yamada KA, Finn MB et al (2005) Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron 48:913–922. doi:10.1016/j.neuron.2005.10.028
De Strooper B, Vassar R, Golde T (2010) The secretases: enzymes with therapeutic potential in Alzheimer disease. Nat Rev Neurol 6:99–107
Delledonne A, Kouri N, Reinstatler L et al (2009) Development of monoclonal antibodies and quantitative ELISAs targeting insulin-degrading enzyme. Mol Neurodegener 4:39. doi:10.1186/1750-1326-4-39
Dickson DW, Crystal HA, Mattiace LA et al (1992) Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging 13:179–189
Eckman EA, Adams SK, Troendle FJ et al (2006) Regulation of steady-state beta-amyloid levels in the brain by neprilysin and endothelin-converting enzyme but not angiotensin-converting enzyme. J Biol Chem 281:30471–30478. doi:10.1074/jbc.M605827200
Fuentealba RA, Liu Q, Zhang J et al (2010) Low-density lipoprotein receptor-related protein 1 (LRP1) mediates neuronal Abeta42 uptake and lysosomal trafficking. PLoS One 5:e11884. doi:10.1371/journal.pone.0011884
Goedert M (1987) Neuronal localization of amyloid beta protein precursor mRNA in normal human brain and in Alzheimer’s disease. EMBO J 6:3627–3632
Goulinet S, Chapman MJ (1993) Plasma lipoproteins in the golden Syrian hamster (Mesocricetus auratus): heterogeneity of apoB- and apoA-I-containing particles. J Lipid Res 34:943–959
Gupta VB, Laws SM, Villemagne VL et al (2011) Plasma apolipoprotein E and Alzheimer disease risk: the AIBL study of aging. Neurology 76:1091–1098
Haddy N, De Bacquer D, Chemaly MM et al (2002) The importance of plasma apolipoprotein E concentration in addition to its common polymorphism on inter-individual variation in lipid levels: results from Apo Europe. Eur J Hum Genet 10:841–850
Holtzman DM, Fagan AM, Mackey B et al (2000) Apolipoprotein E facilitates neuritic and cerebrovascular plaque formation in an Alzheimer’s disease model. Ann Neurol 47:739–747
Iwata N, Tsubuki S, Takaki Y et al (2001) Metabolic regulation of brain Abeta by neprilysin. Science 292:1550–1552. doi:10.1126/science.1059946
Jack CR Jr, Knopman DS, Jagust WJ et al (2010) Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9:119–128. doi:10.1016/s1474-4422(09)70299-6
Jack CR Jr, Lowe VJ, Weigand SD et al (2009) Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain 132:1355–1365. doi:10.1093/brain/awp062
Jack CR Jr, Wiste HJ, Vemuri P et al (2010) Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer’s disease. Brain 133:3336–3348
Jiang Q, Lee CY, Mandrekar S et al (2008) ApoE promotes the proteolytic degradation of Abeta. Neuron 58:681–693. doi:10.1016/j.neuron.2008.04.010
Kanekiyo T, Liu CC, Shinohara M, Li J, Bu G (2012) LRP1 in brain vascular smooth muscle cells mediates local clearance of Alzheimer’s amyloid-beta. J Neurosci 32:16458–16465. doi:10.1523/jneurosci.3987-12.2012
Kanekiyo T, Zhang J, Liu Q, Liu CC, Zhang L, Bu G (2011) Heparan sulphate proteoglycan and the low-density lipoprotein receptor-related protein 1 constitute major pathways for neuronal amyloid-beta uptake. J Neurosci 31:1644–1651. doi:10.1523/jneurosci.5491-10.2011
Kim J, Jiang H, Park S et al (2011) Haploinsufficiency of human APOE reduces amyloid deposition in a mouse model of amyloid-beta amyloidosis. J Neurosci 31:18007–18012. doi:10.1523/jneurosci.3773-11.2011
Klunk WE, Price JC, Mathis CA et al (2007) Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J Neurosci 27:6174–6184. doi:10.1523/jneurosci.0730-07.2007
Koffie R, Hyman B, Spires-Jones T (2011) Alzheimer’s disease: synapses gone cold. Molecular Neurodegeneration 6:63
Koffie RM, Hashimoto T, Tai HC et al (2012) Apolipoprotein E4 effects in Alzheimer’s disease are mediated by synaptotoxic oligomeric amyloid-beta. Brain 135:2155–2168
Koffie RM, Meyer-Luehmann M, Hashimoto T et al (2009) Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci USA 106:4012–4017. doi:10.1073/pnas.0811698106
Koistinaho M, Lin S, Wu X et al (2004) Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat Med 10:719–726. doi:10.1038/nm1058
Lazarov O, Lee M, Peterson DA, Sisodia SS (2002) Evidence that synaptically released beta-amyloid accumulates as extracellular deposits in the hippocampus of transgenic mice. J Neurosci 22:9785–9793
Leissring MA, Farris W, Chang AY et al (2003) Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron 40:1087–1093
Marquez-Sterling NR, Lo AC, Sisodia SS, Koo EH (1997) Trafficking of cell-surface beta-amyloid precursor protein: evidence that a sorting intermediate participates in synaptic vesicle recycling. J Neurosci 17:140–151
May P, Rohlmann A, Bock HH et al (2004) Neuronal LRP1 functionally associates with postsynaptic proteins and is required for normal motor function in mice. Mol Cell Biol 24:8872–8883. doi:10.1128/mcb.24.20.8872-8883.2004
Meilandt WJ, Cisse M, Ho K et al (2009) Neprilysin overexpression inhibits plaque formation but fails to reduce pathogenic Abeta oligomers and associated cognitive deficits in human amyloid precursor protein transgenic mice. J Neurosci 29:1977–1986. doi:10.1523/jneurosci.2984-08.2009
Miller BC, Eckman EA, Sambamurti K et al (2003) Amyloid-beta peptide levels in brain are inversely correlated with insulysin activity levels in vivo. Proc Natl Acad Sci USA 100:6221–6226. doi:10.1073/pnas.1031520100
Mooijaart SP, Berbee JF, van Heemst D et al (2006) ApoE plasma levels and risk of cardiovascular mortality in old age. PLoS Med 3:9
Moore AH, O’Banion MK (2002) Neuroinflammation and anti-inflammatory therapy for Alzheimer’s disease. Adv Drug Deliv Rev 54:1627–1656
Moore BD, Chakrabarty P, Levites Y et al (2012) Overlapping profiles of Abeta peptides in the Alzheimer’s disease and pathological aging brains. Alzheimers Res Ther 4(3):18
Morris JC, Roe CM, Xiong C et al (2010) APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol 67:122–131. doi:10.1002/ana.21843
Nalivaeva NN, Beckett C, Belyaev ND, Turner AJ (2012) Are amyloid-degrading enzymes viable therapeutic targets in Alzheimer’s disease? J Neurochem 1:167–185
Okello A, Koivunen J, Edison P et al (2009) Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PIB PET study. Neurology 73:754–760
Page K, Hollister R, Tanzi RE, Hyman BT (1996) In situ hybridization analysis of presenilin 1 mRNA in Alzheimer disease and in lesioned rat brain. Proc Natl Acad Sci USA 93:14020–14024
Pellerin L, Magistretti PJ (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA 91:10625–10629
Pocivavsek A, Burns MP, Rebeck GW (2009) Low-density lipoprotein receptors regulate microglial inflammation through c-Jun N-terminal kinase. Glia 57:444–453. doi:10.1002/glia.20772
Reiman EM, Chen K, Liu X et al (2009) Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci USA 106:6820–6825. doi:10.1073/pnas.0900345106
Riddell DR, Zhou H, Atchison K et al (2008) Impact of apolipoprotein E (ApoE) polymorphism on brain ApoE levels. J Neurosci 28:11445–11453
Shankar GM, Li S, Mehta TH et al (2008) Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med 14:837–842. doi:10.1038/nm1782
Shibata M, Yamada S, Kumar SR et al (2000) Clearance of Alzheimer’s amyloid-ss(1–40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest 106:1489–1499. doi:10.1172/jci10498
Shigematsu K, McGeer PL, McGeer EG (1992) Localization of amyloid precursor protein in selective postsynaptic densities of rat cortical neurons. Brain Res 592:353–357
Sokolow S, Luu SH, Nandy K et al (2012) Preferential accumulation of amyloid-beta in presynaptic glutamatergic terminals (VGluT1 and VGluT2) in Alzheimer’s disease cortex. Neurobiol Dis 45:381–387
Suh J, Lyckman A, Wang L, Eckman EA, Guenette SY (2011) FE65 proteins regulate NMDA receptor activation-induced amyloid precursor protein processing. J Neurochem 119:377–388
Sullivan PM, Han B, Liu F et al (2011) Reduced levels of human apoE4 protein in an animal model of cognitive impairment. Neurobiol Aging 32:791–801
Takahashi RH, Milner TA, Li F et al (2002) Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol 161:1869–1879
Takami K, Terai K, Matsuo A, Walker DG, McGeer PL (1997) Expression of presenilin-1 and -2 mRNAs in rat and Alzheimer’s disease brains. Brain Res 748:122–130
Thal DR (2012) The role of astrocytes in amyloid beta-protein toxicity and clearance. Exp Neurol 236:1–5
Thal DR, Rub U, Orantes M, Braak H (2002) Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 58:1791–1800
Thinakaran G, Koo EH (2008) Amyloid precursor protein trafficking, processing, and function. J Biol Chem 283:29615–29619. doi:10.1074/jbc.R800019200
Vassar R, Bennett BD, Babu-Khan S et al (1999) Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286:735–741
Verges DK, Restivo JL, Goebel WD, Holtzman DM, Cirrito JR (2011) Opposing synaptic regulation of amyloid-beta metabolism by NMDA receptors in vivo. J Neurosci 31:11328–11337
Villemagne VL, Ataka S, Mizuno T et al (2009) High striatal amyloid beta-peptide deposition across different autosomal Alzheimer disease mutation types. Arch Neurol 66:1537–1544. doi:10.1001/archneurol.2009.285
Villemagne VL, Pike KE, Chetelat G et al (2011) Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann Neurol 69:181–192
Vlassenko AG, Vaishnavi SN, Couture L et al (2010) Spatial correlation between brain aerobic glycolysis and amyloid-beta (Abeta) deposition. Proc Natl Acad Sci USA 107:17763–17767. doi:10.1073/pnas.1010461107
Wenk GL (2003) Neuropathologic changes in Alzheimer’s disease. J Clin Psychiatry 9:7–10
Acknowledgments
We thank Dr. Pritam Das for ELISA reagents detecting Aβ and an antibody against C-terminus region of APP, Drs. Malcolm Leissring and Samir Abdul-Hay for ELISA reagents detecting IDE, Mr. John Gonzalez for assisting with dissection of brain tissues, Ms. Caroline Stetler for careful reading of this manuscript, and Dr. Takahisa Kanekiyo for helpful discussion. This research was supported by grants from the National Institutes of Health (NIH) (P01 AG030128-Project 3 & P01 NS074969-Project 3 to G.B.); Alzheimer’s Drug Discovery Foundation (ADDF) (to G.B.); American Health Assistance Foundation (AHAF) (to G.B.); Mayo Clinic Alzheimer’s Disease Research Center (ADRC) (P50 AG016574) (to D.W.D and M.S.); Japan Heart Foundation and Naito Foundation (to M.S.). The authors also acknowledge the many individuals who contribute to the Mayo Clinic Alzheimer Disease Research Center (PI: R.C.P., P50 AG016574) and Mayo Clinic Study on Aging (PI: R.C.P., U01 AG006786), as well as the neuropathology core in Rochester, MN (Dr. Joseph Parisi), without whose contributions this study would not have been possible.
Conflict of interest
R.C.P. has been chair of a safety monitoring committee for Pfizer (Wyeth) and Janssen Alzheimer Immunotherapy (Elan); and a consultant for Elan Pharmaceuticals and GE Healthcare. All other authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shinohara, M., Petersen, R.C., Dickson, D.W. et al. Brain regional correlation of amyloid-β with synapses and apolipoprotein E in non-demented individuals: potential mechanisms underlying regional vulnerability to amyloid-β accumulation. Acta Neuropathol 125, 535–547 (2013). https://doi.org/10.1007/s00401-013-1086-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-013-1086-9