Abstract
Frontotemporal lobar degeneration with TDP-43 immunoreactive (TDP-ir) inclusions (FTLD-TDP) is sub-classified based on the pattern of neocortical pathology, with each subtype showing clinical and genetic correlations. Recent studies indicate that accurate subtyping of cases may be important to help identify genetic risk factors and develop biomarkers. Although most FTLD-TDP cases are easily classified, some do not match well to one of the existing subtypes. In particular, cases with the C9orf72 repeat expansion (C9+) have been reported to show FTLD-TDP type A, type B or a combination of A and B pathology (A + B). In our series of FTLD-TDP cases, we found that those lacking the C9orf72 mutation (non-C9) were all readily classified as type A (n = 29), B (n = 16) or C (n = 18), using current criteria and standard observational methods. This classification was validated using non-biased hierarchical cluster analysis (HCA) of neocortical pathology data. In contrast, only 14/28 (50%) of the C9+ cases were classified as either pure type A or pure type B, with the remainder showing A + B features. HCA confirmed separation of the C9+ cases into three groups. We then investigated whether patterns of subcortical TDP-ir pathology helped to classify the difficult cases. For the non-C9 cases, each subtype showed a consistent pattern of subcortical involvement with significant differences among the groups. The most distinguishing features included white matter threads, neuronal intranuclear inclusions in hippocampus and striatum, and delicate threads in CA1 in type A; glial cytoplasmic inclusions in white matter and neuronal cytoplasmic inclusions (NCI) in lower motor neurons in type B; compact NCI in striatum in type C. HCA of the C9+ cases based on subcortical features increased the number that clustered with the non-C9 type A (46%) or non-C9 type B (36%); however, there remained a C9+ group with A + B features (18%). These findings suggest that most FTLD-TDP cases can be classified using existing criteria and that each group also shows characteristic subcortical TDP-ir pathology. However, C9+ cases may be unique in the degree to which their pathology overlaps between FTLD-TDP types A and B.
Similar content being viewed by others
Introduction
The neuropathology underlying clinical frontotemporal dementia (FTD) is heterogeneous and is generically referred to as frontotemporal lobar degeneration (FTLD) [6, 24]. The vast majority of FTLD cases can be assigned to one of three major pathological groups, based on the identity of the protein that forms abnormal aggregates within cells. In most series, FTLD with cellular inclusions composed of the transactive response DNA binding protein with Mr 43 kD (TDP-43, FTLD-TDP) is most common [6, 7, 24, 34]. Cases with FTLD-TDP can be further sub-classified based on the relative abundance of different types of TDP-43 immunoreactive (TDP-ir) neuronal inclusions (neuronal cytoplasm inclusions, NCI; neuronal intranuclear inclusions, NII; dystrophic neurites, DN) and their laminar distribution within the cerebral neocortex [7, 22, 26, 41]. The current FTLD-TDP subtyping system has gained wide acceptance and its significance is supported by the fact that each subtype shows relatively specific clinical and genetic correlations. Furthermore, recent studies have demonstrated that cases with each of the different pathological subtypes are associated with different genetic risk factors and that the insoluble protein extracted from postmortem brain tissue has differing physical and biochemical properties [18, 37]. This suggests that accurate pathological subtyping of cases will likely be important to advance the development of biomarkers and targeted therapies for FTD.
Despite the popularity of the current classification system, there remain a number of limitations, with some FTLD-TDP cases proving difficult to classify, either because the pattern of pathology is felt not to fit with any of the existing subtypes [1, 20, 36] or because it shows overlapping features of more than one subtype [1, 2, 35, 42]. Furthermore, studies that have examined the inter-observer reliability have found relatively poor agreement in the subtype assigned to cases, even by experienced neuropathologists [1, 45]. These issues seem to be particularly problematic when attempting to distinguish the two most common FTLD-TDP subtypes, A versus B [1, 2, 45]. In a recent study, we found that qualitative differences in the morphology of the TDP-ir inclusions may be particularly important in differentiating these subtypes; with type A cases characterized by compact NCI (cNCI) and short thick DN, while type B have a predominance of diffuse granular NCI (dNCI) and wispy threads in the cortex [25]. However, even when we employed these refined subtype criteria, we still found a significant proportion of our series that could not easily be classified because they seemed to have a combination of type A and type B features (A + B). Interestingly, 80% of these A + B cases were known to have the C9orf72 repeat expansion (C9+) [8, 39] and this pattern of pathology was present in half of all our C9+ cases, suggesting that there may be something unique about the mechanism(s) by which the C9orf72 mutation leads to TDP mis-metabolism and results in a more heterogeneous pathological signature.
In this study, we have taken two complimentary approaches in an attempt to refine the FTLD-TDP subtype criteria and better classify the difficult cases, including those with the C9orf72 mutation. First, we apply hierarchical cluster analysis (HCA) as a non-biased way of validating the current neocortical pathological criteria and to determine the best fit for the challenging cases. Second, we investigate the patterns of TDP-ir pathology outside the cerebral neocortex to see whether each of the cortical subtypes has a characteristic subcortical pathological signature and whether this additional information could improve case classification. For simplicity sake, we use the term “subcortical” (rather than non-neocortical or extra-neocortical) even though we included pathology in the limbic cortical regions.
Materials and methods
Cases
All cases with a clinical history of dementia and a primary pathological diagnosis of FTLD-TDP from the University of British Columbia research brain bank were considered for the study. Cases were excluded if there was insufficient neocortical TDP-43 pathology to allow for subtype classification (as seen in some cases of ALS with mild dementia) or if tissue blocks representing several of the anatomical areas of interest were not available for examination. We also excluded cases from two families, each with a unique genetic basis; one with a TBK1 mutation [14] and one with a TIA1 mutation [27]. A total of 91 cases were selected, including 20 that also fulfilled clinical criteria for ALS and 48 with a pathogenic mutation in a known FTLD-TDP-causing gene; 20 GRN mutations and 28 with the C9orf72 mutation (C9+) (Table 1). No cases with VCP mutations were available for this analysis. Eighteen (20%) of these cases had an additional chronic co-pathology that, in most cases, primarily affected the cerebral cortex (72% Alzheimer’s type pathology, 17% Lewy body disease) (Table 1).
Immunohistochemistry
All sections were immunostained using a primary antibody that recognizes phosphorylation-independent TDP-43 (ProteinTech Group anti-TARDBP; 1:1000). For quality assurance purposes, select sections were also stained using an antibody that is specific for pathological TDP-43, phosphorylated at site S409/410 (pTDP-43) (clone 1D3, dilution 1:1000) [32] which revealed comparable TDP-ir pathology to the phosphorylation-independent TDP-43 antibody; therefore, we focused all our analysis on data obtained using the phosphorylation-independent antibody. IHC was performed on 5-μm-thick sections of formalin-fixed, paraffin-embedded tissue using the Ventana BenchMark® XT automated staining system (Ventana, Tucson, AZ), following heat-induced antigen retrieval, and developed with either aminoethylcarbizole (AEC) or with the optiVIEW DAB detection kit (DAB).
FTLD-TDP subtypes
Each case was initially assigned an FTLD-TDP subtype, based on standard observational methods, using the current criteria [26] and including the minor modifications recently recommended [25] (hereafter referred to as “standard methods” or “standard assessment”). A designation of FTLD-TDP type A was based on the presence of moderate/abundant cNCI and moderate/abundant short DN concentrated in neocortical layer II and was further supported by the presence of lentiform NII. Type B was assigned to cases with moderate/abundant NCI in all neocortical layers with significantly fewer DN, and was further supported by a predominance of dNCI. Type C cases had many long thick DN with few NCI. Cases were assigned more than one subtype if they had the corresponding positive features of both subtypes; specifically, A + B was used for cases that had cNCI, short DN and NII in layer II (type A features), but also had at least moderate NCI in deep laminae with a significant proportion of NCI (either layer II or deep) being diffuse (type B features).
Semi-quantitative grading of pathology
Grading of the pathology was performed blinded to the clinical and genetic information and to the FTLD-TDP subtype that had been assigned using standard methods. The anatomical regions evaluated included prefrontal neocortex corresponding to Brodmann area 9 (for standard cortical subtyping), and seven additional areas (collectively referred to as “subcortical”), selected based on prior reports of frequent involvement with TDP-ir pathology [13, 15, 16, 23, 33]: the subcortical white matter (evaluated in the same section as used for cortical subtyping), hippocampal dentate granule cell layer, hippocampal CA1 region, striatum, substantia nigra, hypoglossal nucleus and ventral gray matter of the spinal cord. In the frontal cortex, pathological changes were scored separately in the superficial (layer II) and deep (layers IV–VI) laminae. The different types of TDP-ir pathological changes evaluated included compact neuronal cytoplasmic inclusions (cNCI), diffuse granular NCI (dNCI), filamentous NCI (fNCI), neuronal intranuclear inclusions (NII), short thick dystrophic neurites (DN), long thick DN, delicate wispy threads, combined threads and dot-like profiles (ThD), white matter threads and glial cytoplasmic inclusions (GCI). Loss of the normal physiological nuclear staining for TDP-43 was a consistent feature of neurons and glial cells that harbored inclusions in all of the FTLD-TDP subtypes; however, this change was not evaluated as an independent feature. Each type of pathology was graded semi-quantitatively: 0, absent; 1, mild/occasional (easy to find but not present in every medium power field); 2 moderate (at least a few in most fields); 3, severe/abundant (many in virtually every field). In sections of hypoglossal nucleus and spinal cord, a score of 0.5 was assigned when a single lower motor neuron (LMN) NCI was identified in an entire tissue section. In addition to scoring each type of pathology independently, a cumulative grade was assigned for the total amount of TDP-ir pathology in each anatomical region.
Statistical analysis
Statistical analysis was performed with the GraphPad Prism software (Prism 8 for macOS, version 8.1.2). Group comparisons for age at onset, age at death and disease duration were analyzed by t test or one-way analysis of variance (ANOVA) with Tukey’s post hoc test. Nonparametric Kruskal–Wallis (ANOVA) followed by Dunn’s post hoc test or Mann–Whitney U test was used to assess differences in inclusion scores between subgroups. Significance level was set at p < 0.05 (two sided).
Hierarchical cluster analysis (HCA)
HCA of scores for distinct TDP-ir inclusion types in examined regions was carried out and visualized with the online tool Morpheus (https://software.broadinstitute.org/morpheus) using the “one minus Pearson correlation coefficient” metrics with the average linkage method. For TDP-ir pathology in LMN, the higher value of either CN XII or SC total NCI score was used. To analyze the similarity of C9+ cases to prototypic type A and type B features, “type A” and “type B” were included in the analysis which represent median scores of all non-C9 type A and type B cases, respectively. The prototypic features included all of the distinct inclusion types with p < 0.001 in ANOVA and significant differences between type A and type B cases in post hoc test.
Results
Neocortical pathology in non-C9 cases
Based on standard assessment, it was felt that all 63 non-C9 cases could be assigned to a single FTLD-TDP subtype: 29 type A, 16 type B and 18 type C (Table 1). Each of the subtype groups showed the expected genetic and clinical correlations; specifically, all cases with a GRN mutation were type A, the type B cases included all of the non-C9 cases with clinical ALS, and the type C cases included most (77%) of those with the clinical FTD subtype of semantic dementia (svPPA). The neocortical pathology of cases with GRN mutations did not differ significantly from the other type A cases; therefore, these cases were combined as a single type A group for the remainder of the analyses.
Analysis of the semi-quantitative scores confirmed that there were consistent intra-group pathological patterns and clear inter-group differences in the relative amounts and laminar distribution of neocortical pathologies among subtypes A, B and C that matched the subtype criteria (Table 2). Specifically, type A cases had significantly more cNCI, NII and short DN in layer II than the other two groups, whereas type B had more dNCI in both superficial and deep layers, and type C had more long DN. HCA using the neocortical pathological data further confirmed that the non-C9 cases represented three distinct groups that matched perfectly with the classification based on standard assessment and provided independent validation of most discriminating pathological features (Fig. 1a).
Hierarchical cluster analysis of semi-quantitative neocortical pathology data of non-C9 (a) and C9+ cases (b). a Non-C9 cases cluster into three distinct groups that perfectly match the subtype classifications assigned using standard observational methods (Table 1). bC9+ cases split into three groups: one that clusters with non-C9 type A features, one with non-C9 type B features, and a third with positive features of both type A and type B (A + B)
Neocortical pathology in C9+ cases
Based on standard assessment, only 14 (50%) of the C9+ cases could be readily assigned to a single FTLD-TDP subtype, 4 type A and 10 type B; whereas, the remaining 14 cases were felt to have a combination of type A and B pathology (Table 1). HCA confirmed that the C9+ cases split into three groups, one that clustered with the type A features, another which clustered with type B features and a third with the features of both type A and B (Fig. 1b). The grouping based on HCA matched the standard classification, with the exception that one case (case 84) that was classified as A + B based on standard assessment was included in the type A cluster. HCA also showed that the group with type B features was most distinct and that the A + B cluster was more similar to type A.
Subcortical pathology in non-C9 cases
There were significant differences among the neocortical subtype groups in the total amount of TDP-ir pathology and the abundance of different types of inclusions in each of the subcortical regions evaluated (Table 3, Figs. 2, 3).
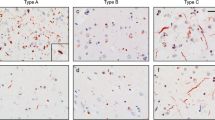
Characteristic TDP-43 immunoreactive pathological features in subcortical regions of non-C9 cases. a–c Cerebral white: moderate threads in type A (a); moderate glial cytoplasmic inclusions (GCI) in type B (b); occasional threads in type C (c). d–f Dentate granule cell layer of hippocampus: moderate neuronal cytoplasmic inclusions (NCI) which are mostly compact (cNCI) and occasional neuronal intranuclear inclusions (NII, inset) in type A (d); abundant compact and diffuse NCI (dNCI, inset) in type B (e); abundant Pick body-like cNCI (inset) in type C (f). g–i CA1 pyramidal layer of hippocampus: abundant delicate threads in type A (g); occasional dNCI in type B (h); occasional long thick dystrophic neurites (DN) in type C (i). j–l Striatum: abundant short DN and occasional NII (inset) in type A (j); abundant dNCI in type B (k); abundant cNCI in type C (l). m–o Substantia nigra: moderate short DN (arrows) and occasional filamentous NCI in type A (m); abundant dNCI and occasional cNCI and GCI in type B (n); occasional long thick DN in type C (o). p–r Hypoglossal nucleus and ventral gray matter of spinal cord: rare DN in type A (arrow, p); abundant NCI in type B (q); rare DN in type C (arrow, r). Bar: 50 µm (a–o), 20 µm (d-f, inset), 80 µm (p–r)
Subcortical TDP-43 immunoreactive pathology (TDP-ir) in non-C9 cases. There were significant differences among the neocortical FTLD-TDP subtypes in the total amount of TDP-ir pathology and the abundance of different types of inclusions in each of the subcortical regions evaluated. CA1, cornu ammonis region 1 of hippocampus; cNCI, compact neuronal cytoplasmic inclusions; CN XII, hypoglossal nucleus; DN, dystrophic neurites; dNCI, diffuse NCI; fNCI, filamentous NCI; GCI, glial cytoplasmic inclusions; NII, neuronal intranuclear inclusions; ThD, delicate short threads and dots. #, significantly different (greater or lesser) than other two groups. Graphs show median with 95% confidence interval
Cerebral white matter
Type A cases had more cerebral white matter pathology than the other groups, consisting predominantly of short thread-like processes. In contrast, in type B cases, the white matter pathology was almost exclusively GCI. Type C cases had significantly less white matter pathology than the other two groups.
Hippocampal dentate
In all three subtype groups, the predominant type of inclusion in the dentate granule cells was cNCI. Type A cases had significantly less total pathology in the hippocampal dentate, but was the only group with any NII (61% of cases). Type B cases had significantly more dNCI, which were almost as abundant as cNCI. In type C cases, the NCI were almost exclusively compact and often had solid round “Pick body-like” morphology.
Hippocampal CA1
One of the most striking findings was that delicate wispy threads in CA1 were an absolutely sensitive and specific feature of type A cases. Furthermore, this CA1 thread pathology was graded as moderate or abundant in all type A cases, with the exception of three cases in which mild thread pathology was associated with complete loss of pyramidal neurons (end-stage hippocampal sclerosis, data not shown). NCI in CA1 neurons were most abundant in type B cases while long thick DN were most abundant in type C.
Striatum
All of the subtype groups had abundant pathology in the striatum, but with significant differences in the specific inclusion types: type A cases had more DN and were the only group with any NII (in 90% of cases), type B had more dNCI and GCI, while type C had more cNCI, which again resembled small Pick bodies.
Substantia nigra
Type B cases had the most pathology, including significantly more dNCI, cNCI and GCI, while type A case had more DN and fNCI. Type C cases never showed more than mild DN pathology.
Hypoglossal nucleus and spinal cord
Type B cases had significantly more pathology, including all LMN NCI morphologies and GCI. Type A and C cases rarely showed more than occasional small DN.
HCA based on the subcortical pathology data confirmed that the non-C9 cases separated into three distinct groups that perfectly matched the neocortical subtypes (Fig. 4a).
Hierarchical cluster analysis of semi-quantitative subcortical pathology data of non-C9 (a) and C9+ cases (b). a Non-C9 cases cluster into three distinct groups that perfectly match the subtype classifications based on neocortical data. bC9+ cases cluster into three groups. Cases with pure type B neocortical pathology also cluster with non-C9 type B based on subcortical features. The other cases cluster with the type A non-C9 cases based on their subcortical features, with a small sub-cluster (A + B) characterized by additional LMN pathology (asterisk) as a prototypic subcortical type B feature
Subcortical pathology in C9+ cases
HCA of the C9+ cases based on their subcortical pathological features again showed separation into three groups (Fig. 4b). The ten cases with pure type B neocortical pathology also clustered with non-C9 type B subcortical features. The other C9+ cases with A or A + B neocortical features were found to cluster with the non-C9 type A cases based on analysis of their subcortical features, with five cases forming a sub-cluster (A + B) that differed only by having moderate or abundant NCI in LMN (p < 0.001), typical of type B.
Overall classification and clinical correlations of C9+ cases
Evaluation of both the neocortical and subcortical data showed that the C9+ cases fell into four subgroups: cases with pure type A cortical and subcortical features (4/28 = 14%), pure type B cortical and subcortical features (10/28 = 36%), and two more closely related groups, both with A + B cortical and type A subcortical features, that differed only in the degree of type B LMN pathology (Table 4).
All of the C9+ cases with clinical ALS had type B cortical and subcortical pathology (either pure type B or A + B). Compared to cases with type A features (either pure A or A + B), those with pure type B were younger at death (56 ± 8 years vs. 68 ± 11 years, p = 0.006) and had a shorter disease duration (3 ± 2 vs. 10 ± 4 years, p = 0.0002). Interestingly, the shorter duration of the pure type B cases was only partially explained by the fact that 50% had also suffered from clinical ALS since even the type B cases with pure FTD had a shorter duration than those with FTD and type A pathology (4 ± 3 vs. 10 ± 4 years, p = 0.009). The group of five cases that had combined type A and type B cortical and subcortical pathology were somewhat older at onset than the other groups; however, this difference was not significant.
Discussion
In the large cohort of FTLD-TDP cases investigated in this study, we found that all our non-C9 cases could be readily classified as one of the three common subtypes, based on current pathological criteria and standard methods or assessment [26]. Furthermore, each of the subtypes showed the expected clinical and genetic correlations. HCA of the non-C9 cases using semi-quantitative neocortical pathology data provided independent validation of the current classification methods and criteria. However, as with our previous study [25], we found that our C9+ cases did not show a consistent single pathological subtype using the standard methods, but included cases that appeared to be pure type A, pure type B or have a combination of type A and B features. Although the degree of pathological heterogeneity in our C9+ group is somewhat surprising, given the consistent genetic–pathological correlations that are seen with most other FTLD-TDP causing mutations (type A with GRN, type B with TIA1, type D with VCP) [10, 23, 27], it is not unprecedented; although the number of reports describing the pathology associated with TBK1 mutations is still relatively few, they include cases with either type A or type B FTLD-TDP [12, 14, 17, 19, 49].
It is worth noting that neither standard analysis nor HCA identified any cases with other unique subtypes or combinations of subtypes, within our cohort. Specifically, we did not find any cases where type C features were combined with either A or B, as has been reported in another study [35]. More importantly, the HCA failed to identify a distinct cluster that would correspond to cases previously proposed to represent the novel subtype designated as type E [20] despite the fact that our analysis included the characteristic cortical and subcortical features (dNCI, ThD and GCI). This finding does not exclude the existence of type E cases, but indicates that they were not present in our cohort as a distinct subset.
Most previous studies have also reported that the TDP-ir pathology associated with the C9orf72 mutation does not match consistently to a single specific FTLD-TDP subtype. Although a few groups have described small series of C9+ cases with consistent FTLD-TDP type B pathology [9, 11, 43], most have reported their cohorts to include some cases with pure type A pathology and others with pure type B [3, 4, 28,29,30, 35, 44]. Only a few studies have specifically described C9+ cases with a combination of type A and B features [35, 42]; however, the true frequency of such cases may be under-reported. Although the original papers [22, 41] and subsequent consensus diagnostic criteria [6, 26] do not explicitly state that a case cannot have the features of more than one FTLD-TDP subtype, this is implied by the dichotomous nature of the inclusion and exclusion criteria. As a result, it is likely that investigators may feel obliged to assign cases with overlapping pathology to a single existing subtype by arbitrarily deciding which combination of features has priority over the others, weighing the relative diagnostic importance of the positive (inclusion) and negative (exclusion) criteria. The potential bias in this type of subjective classification may explain the poor inter-observer agreement that has been reported, particularly when distinguishing type A and type B cases [1, 2, 45].
In an attempt to reduce the subjectivity involved in classifying our C9+ cases, we performed HCA, comparing the semi-quantitative neocortical pathology data against the features that best separated our non-C9 type A from our non-C9 type B cases. Although this analysis confirmed the presence of subsets of C9+ cases that matched well with pure type B and pure type A, there remained a significant proportion of C9+ cases (46%) that formed a separate cluster with A + B features.
We next evaluated the subcortical TDP-ir pathological features of our non-C9 cases to see whether each subtype had a characteristic subcortical signature that might help in classifying cases with ambiguous cortical pathology. We found that each cortical subtype displayed a consistent pattern of subcortical involvement and that there were significant differences among the groups (Figs. 2, 3). HCA confirmed that the non-C9 cases separated into three distinct groups based on the subcortical pathology data. The most characteristic features of type A cases were abundant white matter threads, a predominance of DN in subcortical gray matter regions and small numbers of NII in the hippocampus and striatum (Table 5). In addition, delicate threads in CA1 were only seen in the type A cases and were often associated with significant pyramidal cell loss (hippocampal sclerosis). In contrast, type B cases had a predominance of GCI in the cerebral white matter and the gray matter pathology consisted primarily of NCI which were most often diffuse. Moderate or abundant NCI in LMN was the most defining feature of the type B group. Subcortical pathology in type C cases was more anatomically restricted, with little involvement of the white matter, brainstem or spinal cord. However, in the hippocampal dentate and striatum, the type C cases consistently showed numerous cNCI that had a unique “Pick body-like” morphology with uniform solid consistency and smooth round contour (in contrast to type A and B cases where cNCI usually appeared as a compact aggregate of coarse granules).
A number of previous reports have described TDP-ir pathology in anatomical regions beyond the neocortex in FTLD-TDP; however, most have focused on a single clinical, genetic or pathological group of patients [5, 15, 23]. The study by Josephs et al. was similar to ours in specifically comparing the subcortical pathology among FTLD-TDP subtypes to determine if each has a characteristic pattern [16]. Although the anatomical regions and morphological types of TDP-ir pathological inclusions they evaluated differed slightly from the present study, these authors also found that each cortical subtype had a characteristic subcortical pattern of pathology, and most of their key findings were similar to ours: their type A cases had more DN and NII, NCI were a mix of compact and granular, and delicate neurites in CA1 were “largely restricted” to this group; NCI in type B were mostly diffuse granular “pre-inclusions” and this was the only group with NCI in hypoglossal LMN; type C cases had dense round Pick body-like inclusions and more long thick DN in the amygdala. Perhaps due to the smaller sample sizes in their study (N = 39), the only features that Josephs et al. found to distinguish one group from both the others were hypoglossal NCI in type B, thalamic DN in type A and amygdala DN in type C. Furthermore, it is important to note that the C9orf72 repeat expansion had not yet been identified when that study was performed and that those investigators did not classify any cases as having more than one cortical subtype, meaning that any such cases must have been assigned to what they felt was the best match.
Differences in the anatomical patterns of subcortical involvement may explain some of the additional clinical heterogeneity that exists among the FTLD-TDP subtypes, beyond their association with different FTD phenotypes [24]. Consistent involvement of the hippocampal pyramidal layer in cases with type A pathology provides an explanation for their more common amnestic presentation (Table 1) [16, 21]. This association may also provide important insight into the pathogenesis of hippocampal sclerosis in advanced aging which is frequently associated with similar TDP-ir thread pathology in CA1 [31]. LMN pathology in type B cases is an obvious correlate for the high frequency of ALS. Finally, differences in the burden of pathology in the substantia nigra may explain why parkinsonism is relatively common in patients with type A and type B pathology but not with type C [15, 16, 21]. Although this study was not designed to evaluate the possibility of prion-like spreading of TDP-ir pathology, it is interesting to note that the subgroup with the shortest mean disease duration (type B, 3.7 years) had the most anatomically widespread TDP-ir pathology, while the group with the longest duration (type C, 8.9 years) had the most restricted pathology. This suggests that, if pathological forms of TDP-43 do spread along anatomical pathways, then there must be significant differences in the mechanism of spreading and/or the pathological protein species (i.e., seeds) among the different FTLD-TDP subtypes that influence this propagation.
Subcortical TDP-ir pathological features are not included in the current FTLD-TDP classification criteria; however, the degree to which they match the cortical subtypes suggests that subcortical evaluation might be helpful in classifying cases with ambiguous cortical features (Table 5). HCA of our C9+ cases using the subcortical pathology data confirmed the presence of subsets with pure type A and with pure type B pathology. In addition, it showed that the subcortical pathology of most of the cases with overlapping (A + B) neocortical pathology was similar to pure type A. Nonetheless, even when combining the data from both cortical and subcortical evaluation, there remained a significant group (18%) with a combination of type A and type B features. In interpreting these findings, it is worth emphasizing that HCA did not find that the C9+ cases simply represented a pathological continuum with an even distribution of all combinations of A and B pathology, as suggested by a previous study using principal components analysis [2]; nor did it find evidence for three distinct groups, one with pure type A, another with pure type B and a third with a complete overlap, showing a full complement of all type A and all type B cortical and subcortical features. Instead, the C9+ cases showed an uneven distribution along the pathological spectrum that will have to be accounted for by any proposed model.
These findings suggest that cases with the C9orf72 repeat expansion are somewhat unique, compared with other FTD genetic subtypes, in the degree of associated pathological (and clinical) heterogeneity, which likely reflects a particular susceptibility to the modulating influences of additional genetic or environmental factor(s) [48]. Although there is a broad range in the size of the C9orf72 GGGGCC repeat expansion that is pathogenic, most studies have found little evidence that the specific number of repeats significantly influences the phenotype [40]. Alternatively, variation in other genes could influence both the clinical and pathological expression; for instance, inheritance of the GRN rs5848 T-allele is known to increase the risk for FTLD-TDP with type A pathology [38], while intermediate repeat length in ATXN2 increases the risk for ALS (presumably with type B pathology) and homozygosity of the minor allele of TMEM106B protects C9+ patients from FTD but not from ALS [46, 47]. Evaluation of such genetic modifiers is beyond the scope of the current study and would require a much larger series of C9+ cases with known FTLD-TDP subtype.
In summary, we found that most cases of FTD with TDP-ir pathology can be readily assigned to one of the existing FTLD-TDP subtypes using the current criteria and standard subjective method of evaluation. The validity of this classification is further supported by non-biased HCA of semi-quantitative pathology data and by the striking correlations that exist between patterns of neocortical and subcortical pathology. The different patterns of subcortical involvement may help to explain the range of clinical features associated with each of the pathological subtypes and could be helpful in classifying cases where the neocortical findings are not straightforward. C9+ cases are unique in the degree to which their pathology overlaps between multiple FTLD-TDP subtypes (A and B), suggesting that there may be something novel about the mechanism(s) by which the C9orf72 mutation leads to TDP-43 mis-metabolism and aggregation. We recognize that these findings need to be validated by other research groups in their own cohorts.
References
Alafuzoff I, Pikkarainen M, Neumann M, Arzberger T, Al-Sarraj S, Bodi I et al (2015) Neuropathological assessments of the pathology in frontotemporal lobar degeneration with TDP43-positive inclusions: an inter-laboratory study by the BrainNet Europe consortium. J Neural Transm 122:957–972. https://doi.org/10.1007/s00702-014-1304-1
Armstrong RA, Ellis W, Hamilton RL, Mackenzie IR, Hedreen J, Gearing M et al (2010) Neuropathological heterogeneity in frontotemporal lobar degeneration with TDP-43 proteinopathy: a quantitative study of 94 cases using principal components analysis. J Neural Transm 117:227–239. https://doi.org/10.1007/s00702-009-0350-6
Bigio EH, Weintraub S, Rademakers R, Baker M, Ahmadian SS, Rademaker A et al (2013) Frontotemporal lobar degeneration with TDP-43 proteinopathy and chromosome 9p repeat expansion in C9ORF72: clinicopathologic correlation. Neuropathology 33:122–133. https://doi.org/10.1111/j.1440-1789.2012.01332.x
Boeve BF, Boylan KB, Graff-Radford NR, DeJesus-Hernandez M, Knopman DS, Pedraza O et al (2012) Characterization of frontotemporal dementia and/or amyotrophic lateral sclerosis associated with the GGGGCC repeat expansion in C9ORF72. Brain 135:765–783. https://doi.org/10.1093/brain/aws004
Brandmeir NJ, Geser F, Kwong LK, Zimmerman E, Qian J, Lee VM et al (2008) Severe subcortical TDP-43 pathology in sporadic frontotemporal lobar degeneration with motor neuron disease. Acta Neuropathol 115:123–131. https://doi.org/10.1007/s00401-007-0315-5
Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ et al (2007) Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 114:5–22. https://doi.org/10.1007/s00401-007-0237-2
Cairns NJ, Neumann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ et al (2007) TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol 171:227–240. https://doi.org/10.2353/ajpath.2007.070182
DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ et al (2011) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72:245–256. https://doi.org/10.1016/j.neuron.2011.09.011
Dobson-Stone C, Hallupp M, Bartley L, Shepherd CE, Halliday GM, Schofield PR et al (2012) C9ORF72 repeat expansion in clinical and neuropathologic frontotemporal dementia cohorts. Neurology 79:995–1001. https://doi.org/10.1212/WNL.0b013e3182684634
Forman MS, Mackenzie IR, Cairns NJ, Swanson E, Boyer PJ, Drachman DA et al (2006) Novel ubiquitin neuropathology in frontotemporal dementia with valosin-containing protein gene mutations. J Neuropathol Exp Neurol 65:571–581. https://doi.org/10.1097/00005072-200606000-00005
Gijselinck I, Van Langenhove T, van der Zee J, Sleegers K, Philtjens S, Kleinberger G et al (2012) A C9orf72 promoter repeat expansion in a Flanders-Belgian cohort with disorders of the frontotemporal lobar degeneration-amyotrophic lateral sclerosis spectrum: a gene identification study. Lancet Neurol 11:54–65. https://doi.org/10.1016/S1474-4422(11)70261-7
Gijselinck I, Van Mossevelde S, van der Zee J, Sieben A, Philtjens S, Heeman B et al (2015) Loss of TBK1 is a frequent cause of frontotemporal dementia in a Belgian cohort. Neurology 85:2116–2125. https://doi.org/10.1212/WNL.0000000000002220
Hatanpaa KJ, Bigio EH, Cairns NJ, Womack KB, Weintraub S, Morris JC et al (2008) TAR DNA-binding protein 43 immunohistochemistry reveals extensive neuritic pathology in FTLD-U: a midwest-southwest consortium for FTLD study. J Neuropathol Exp Neurol 67:271–279
Hirsch-Reinshagen V, Alfaify OA, Hsiung GR, Pottier C, Baker M, Perkerson RB III et al (2019) Clinicopathologic correlations in a family with a TBK1 mutation presenting as primary progressive aphasia and primary lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. https://doi.org/10.1080/21678421.2019.1632347
Hsiung GY, DeJesus-Hernandez M, Feldman HH, Sengdy P, Bouchard-Kerr P, Dwosh E et al (2012) Clinical and pathological features of familial frontotemporal dementia caused by C9ORF72 mutation on chromosome 9p. Brain 135:709–722. https://doi.org/10.1093/brain/awr354
Josephs KA, Stroh A, Dugger B, Dickson DW (2009) Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes. Acta Neuropathol 118:349–358. https://doi.org/10.1007/s00401-009-0547-7
Koriath CA, Bocchetta M, Brotherhood E, Woollacott IO, Norsworthy P, Simon-Sanchez J et al (2017) The clinical, neuroanatomical, and neuropathologic phenotype of TBK1-associated frontotemporal dementia: a longitudinal case report. Alzheimers Dement 6:75–81. https://doi.org/10.1016/j.dadm.2016.10.003
Laferriere F, Maniecka Z, Perez-Berlanga M, Hruska-Plochan M, Gilhespy L, Hock EM et al (2019) TDP-43 extracted from frontotemporal lobar degeneration subject brains displays distinct aggregate assemblies and neurotoxic effects reflecting disease progression rates. Nat Neurosci 22:65–77. https://doi.org/10.1038/s41593-018-0294-y
Lamb R, Rohrer JD, Real R, Lubbe SJ, Waite AJ, Blake DJ et al (2019) A novel TBK1 mutation in a family with diverse frontotemporal dementia spectrum disorders. Cold Spring Harb Mol Case Stud. https://doi.org/10.1101/mcs.a003913
Lee EB, Porta S, Michael Baer G, Xu Y, Suh E, Kwong LK et al (2017) Expansion of the classification of FTLD-TDP: distinct pathology associated with rapidly progressive frontotemporal degeneration. Acta Neuropathol 134:65–78. https://doi.org/10.1007/s00401-017-1679-9
Mackenzie IR (2007) The neuropathology and clinical phenotype of FTD with progranulin mutations. Acta Neuropathol 114:49–54. https://doi.org/10.1007/s00401-007-0223-8
Mackenzie IR, Baborie A, Pickering-Brown S, Du Plessis D, Jaros E, Perry RH et al (2006) Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol 112:539–549. https://doi.org/10.1007/s00401-006-0138-9
Mackenzie IR, Baker M, Pickering-Brown S, Hsiung GY, Lindholm C, Dwosh E et al (2006) The neuropathology of frontotemporal lobar degeneration caused by mutations in the progranulin gene. Brain 129:3081–3090. https://doi.org/10.1093/brain/awl271
Mackenzie IR, Neumann M (2016) Molecular neuropathology of frontotemporal dementia: insights into disease mechanisms from postmortem studies. J Neurochem 138(Suppl 1):54–70. https://doi.org/10.1111/jnc.13588
Mackenzie IR, Neumann M (2017) Reappraisal of TDP-43 pathology in FTLD-U subtypes. Acta Neuropathol 134:79–96. https://doi.org/10.1007/s00401-017-1716-8
Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E et al (2011) A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 122:111–113. https://doi.org/10.1007/s00401-011-0845-8
Mackenzie IR, Nicholson AM, Sarkar M, Messing J, Purice MD, Pottier C et al (2017) TIA1 mutations in amyotrophic lateral sclerosis and frontotemporal dementia promote phase separation and alter stress granule dynamics. Neuron 95(808–816):e809. https://doi.org/10.1016/j.neuron.2017.07.025
Mahoney CJ, Beck J, Rohrer JD, Lashley T, Mok K, Shakespeare T et al (2012) Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical, neuroanatomical and neuropathological features. Brain 135:736–750. https://doi.org/10.1093/brain/awr361
Mann DM, Rollinson S, Robinson A, Bennion Callister J, Thompson JC, Snowden JS et al (2013) Dipeptide repeat proteins are present in the p62 positive inclusions in patients with frontotemporal lobar degeneration and motor neurone disease associated with expansions in C9ORF72. Acta Neuropathol Commun 1:68. https://doi.org/10.1186/2051-5960-1-68
Murray ME, Dejesus-Hernandez M, Rutherford NJ, Baker M, Duara R, Graff-Radford NR et al (2011) Clinical and neuropathologic heterogeneity of c9FTD/ALS associated with hexanucleotide repeat expansion in C9ORF72. Acta Neuropathol 122:673–690. https://doi.org/10.1007/s00401-011-0907-y
Nelson PT, Schmitt FA, Lin Y, Abner EL, Jicha GA, Patel E et al (2011) Hippocampal sclerosis in advanced age: clinical and pathological features. Brain 134:1506–1518. https://doi.org/10.1093/brain/awr053
Neumann M, Kwong LK, Lee EB, Kremmer E, Flatley A, Xu Y et al (2009) Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol 117:137–149. https://doi.org/10.1007/s00401-008-0477-9
Neumann M, Kwong LK, Truax AC, Vanmassenhove B, Kretzschmar HA, Van Deerlin VM et al (2007) TDP-43-positive white matter pathology in frontotemporal lobar degeneration with ubiquitin-positive inclusions. J Neuropathol Exp Neurol 66:177–183. https://doi.org/10.1097/01.jnen.0000248554.45456.58
Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT et al (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133. https://doi.org/10.1126/science.1134108
Nishihira Y, Gefen T, Mao Q, Appin C, Kohler M, Walker J et al (2019) Revisiting the utility of TDP-43 immunoreactive (TDP-43-ir) pathology to classify FTLD-TDP subtypes. Acta Neuropathol 138:167–169. https://doi.org/10.1007/s00401-019-02024-w
Pikkarainen M, Hartikainen P, Alafuzoff I (2008) Neuropathologic features of frontotemporal lobar degeneration with ubiquitin-positive inclusions visualized with ubiquitin-binding protein p62 immunohistochemistry. J Neuropathol Exp Neurol 67:280–298. https://doi.org/10.1097/NEN.0b013e31816a1da2
Pottier C, Ren Y, Perkerson RB III, Baker M, Jenkins GD, van Blitterswijk M et al (2019) Genome-wide analyses as part of the international FTLD-TDP whole-genome sequencing consortium reveals novel disease risk factors and increases support for immune dysfunction in FTLD. Acta Neuropathol 137:879–899. https://doi.org/10.1007/s00401-019-01962-9
Rademakers R, Eriksen JL, Baker M, Robinson T, Ahmed Z, Lincoln SJ et al (2008) Common variation in the miR-659 binding-site of GRN is a major risk factor for TDP43-positive frontotemporal dementia. Hum Mol Genet 17:3631–3642. https://doi.org/10.1093/hmg/ddn257
Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR et al (2011) A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72:257–268. https://doi.org/10.1016/j.neuron.2011.09.010
Rutherford NJ, Heckman MG, Dejesus-Hernandez M, Baker MC, Soto-Ortolaza AI, Rayaprolu S et al (2012) Length of normal alleles of C9ORF72 GGGGCC repeat do not influence disease phenotype. Neurobiol Aging 33(2950):e2955–e2957. https://doi.org/10.1016/j.neurobiolaging.2012.07.005
Sampathu DM, Neumann M, Kwong LK, Chou TT, Micsenyi M, Truax A et al (2006) Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol 169:1343–1352. https://doi.org/10.2353/ajpath.2006.060438
Shinagawa S, Naasan G, Karydas AM, Coppola G, Pribadi M, Seeley WW et al (2015) Clinicopathological study of patients with C9ORF72-associated frontotemporal dementia presenting with delusions. J Geriatr Psychiatry Neurol 28:99–107. https://doi.org/10.1177/0891988714554710
Simon-Sanchez J, Dopper EG, Cohn-Hokke PE, Hukema RK, Nicolaou N, Seelaar H et al (2012) The clinical and pathological phenotype of C9ORF72 hexanucleotide repeat expansions. Brain 135:723–735. https://doi.org/10.1093/brain/awr353
Snowden JS, Rollinson S, Thompson JC, Harris JM, Stopford CL, Richardson AM et al (2012) Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain 135:693–708. https://doi.org/10.1093/brain/awr355
Tan RH, Shepherd CE, Kril JJ, McCann H, McGeachie A, McGinley C et al (2013) Classification of FTLD-TDP cases into pathological subtypes using antibodies against phosphorylated and non-phosphorylated TDP43. Acta Neuropathol Commun 1:33. https://doi.org/10.1186/2051-5960-1-33
van Blitterswijk M, Mullen B, Heckman MG, Baker MC, DeJesus-Hernandez M, Brown PH et al (2014) Ataxin-2 as potential disease modifier in C9ORF72 expansion carriers. Neurobiol Aging 35(2421):e2413–e2427. https://doi.org/10.1016/j.neurobiolaging.2014.04.016
van Blitterswijk M, Mullen B, Nicholson AM, Bieniek KF, Heckman MG, Baker MC et al (2014) TMEM106B protects C9ORF72 expansion carriers against frontotemporal dementia. Acta Neuropathol 127:397–406. https://doi.org/10.1007/s00401-013-1240-4
van Blitterswijk M, Mullen B, Wojtas A, Heckman MG, Diehl NN, Baker MC et al (2014) Genetic modifiers in carriers of repeat expansions in the C9ORF72 gene. Mol Neurodegener 9:38. https://doi.org/10.1186/1750-1326-9-38
van der Zee J, Gijselinck I, Van Mossevelde S, Perrone F, Dillen L, Heeman B et al (2017) TBK1 mutation spectrum in an extended european patient cohort with frontotemporal dementia and amyotrophic lateral sclerosis. Hum Mutat 38:297–309. https://doi.org/10.1002/humu.23161
Acknowledgements
We would like to thank Margaret Luk, Simon Cheung, and Manuel Gödan for their excellent technical assistance. This work was supported by the German Helmholtz Association (W2/W3-036, MN) and the Canadian Institutes of Health Research (74580, IRM).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mackenzie, I.R., Neumann, M. Subcortical TDP-43 pathology patterns validate cortical FTLD-TDP subtypes and demonstrate unique aspects of C9orf72 mutation cases. Acta Neuropathol 139, 83–98 (2020). https://doi.org/10.1007/s00401-019-02070-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-019-02070-4