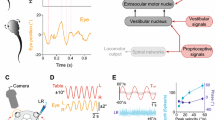
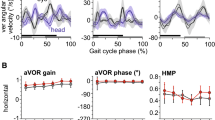
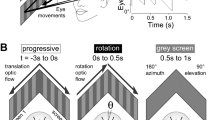
Abstract
In guiding adaptive behavior, efference copy signals or corollary discharge are traditionally considered to serve as predictors of self-generated sensory inputs and by interfering with their central processing are able to counter unwanted consequences of an animal’s own actions. Here, in a speculative reflection on this issue, we consider a different functional role for such intrinsic predictive signaling, namely in stabilizing gaze during locomotion where resultant changes in head orientation in space require online compensatory eye movements in order to prevent retinal image slip. The direct activation of extraocular motoneurons by locomotor-related efference copies offers a prospective substrate for assisting self-motion derived sensory feedback, rather than being subtracted from the sensory signal to eliminate unwanted reafferent information. However, implementing such a feed-forward mechanism would be critically dependent on an appropriate phase coupling between rhythmic propulsive movement and resultant head/visual image displacement. We used video analyzes of actual locomotor behavior and basic theoretical modeling to evaluate head motion during stable locomotion in animals as diverse as Xenopus laevis tadpoles, teleost fish and horses in order to assess the potential suitability of spinal efference copies to the stabilization of gaze during locomotion. In all three species, and therefore regardless of aquatic or terrestrial environment, the head displacements that accompanied locomotor action displayed a strong correlative spatio-temporal relationship in correspondence with a potential predictive value for compensatory eye adjustments. Although spinal central pattern generator-derived efference copies offer appropriately timed commands for extraocular motor control during self-generated motion, it is likely that precise image stabilization requires the additional contributions of sensory feedback signals. Nonetheless, the predictability of the visual consequences of stereotyped locomotion renders intrinsic efference copy signaling an appealing mechanism for offsetting these disturbances, thus questioning the exclusive role traditionally ascribed to sensory-motor transformations in stabilizing gaze during vertebrate locomotion.
Similar content being viewed by others
References
Angelaki DE, Cullen KE (2008) Vestibular system: the many facets of a multimodal sense. Annu Rev Neurosci 31: 125–150
Angelaki DE, Hess BJ (2005) Selfmotion-induced eye movements: effects on visual acuity and navigation. Nat Rev Neurosci 6: 966–976
Azizi E, Landberg T, Wassersug RJ (2007) Vertebral function during tadpole locomotion. Zoology 110(4): 290–297 doi:10.1016/j.zool.2007.02.002
Bell C (1981) An efference copy which is modified by reafferent input. Science 214: 450–453
Beyeler A, Métais C, Combes D, Simmers J, Le Ray D (2008) Metamorphosis-induced changes in the coupling of spinal thoraco-lumbar motor outputs during swimming in Xenopus laevis. J Neurophys 100(3): 1372–1383
Combes D, Merrywest SD, Simmers J, Sillar KT (2004) Developmental segregation of spinal networks driving axial- and hindlimb-based locomotion in metamorphosing Xenopus laevis. J Physiol 559(1): 17–24
Combes D, Le Ray D, Lambert FM, Simmers J, Straka H (2008) An intrinsic feed-forward mechanism for vertebrate gaze stabilization. Curr Biol 18: R241–243
Crapse TB, Sommer MA (2008) Corollary discharge across the animal kingdom. Nat Rev Neurosci 9(8): 587–600
Cullen KE (2004) Sensory signals during active versus passive movement. Curr Opin Neurobiol 14: 698–706
Dietz V (1992) Human neuronal control of automatic functional movements: interaction between central programs and afferent input. Physiol Rev 72: 33–69
Easter SS, Johns PR (1974) Horizontal compensatory eye movements in goldfish (Carassius auratus). J Comp Physiol A 92(1): 37–57 doi:10.1007/bf00696525
Gittis AH, du Lac S (2006) Intrinsic and synaptic plasticity in the vestibular system. Curr Opin Neurobiol 16(4): 385–390 doi:10.1016/j.conb.2006.06.012
Grillner S (1981) Control of locomotion in bipeds, tetrapods, and fish. In: Brookhart JM, Mountcastle VB (eds) Handbook of physiology—the nervous system II. American Physiological Society, Bethesda, pp 1179–1236
Grillner S (2006) Biological pattern generation: the cellular and computational logic of networks in motion. Neuron 52(5): 751–766 doi:10.1016/j.neuron.2006.11.008
Harris LR (1965) Visual motion caused by movements of the eye, head and body. In: Smith AT, Snowman RJ (eds) Visual detection of motion. Academic Press, London, pp 397–435
Kheradmand A, Zee DS (2011) Cerebellum and ocular motor control. Frontiers Neurol 2. doi:10.3389/fneur.2011.00053
Lambert FM, Combes D, Simmers J, Straka H (2012) Gaze stabilization by efference copy signaling without sensory feedback during vertebrate locomotion. Curr Biol 22: 1649–1658
Lyon EP (1900) Compensatory motion in fishes. Am J Physiol 4(2): 77–82
Marlinsky VV (1992) Activity of lateral vestibular nucleus neurons during locomotion in the decerebrate Guinea pig. Exp Brain Res 90(3): 583–588. doi:10.1007/bf00230942
Montgomery JC, Bodznick D (1994) An adaptive filter that cancels self-induced noise in the electrosensory and lateral line mechanosensory systems of fish. Neurosci Lett 174(2): 145–148
Orlovsky GN (1972) Activity of vestibulospinal neurons during locomotion. Brain Res 46(0): 85–98 doi:10.1016/0006-8993(72)90007-8
Orlovsky GN, Deliagina TG, Grillner S (1999) Neuronal control of locomotion: from mollusc to man. Oxford University Press, New York
Poulet JFA, Hedwig B (2007) New insights into corollary discharges mediated by identified neural pathways. Trends Neurosci 30(1): 14–21
Roberts BL, Russell IJ (1972) The activity of lateral-line efferent neurones in stationary and swimming dogfish. J Exp Biol 57(2): 435–448
Rossignol S (1996) Visuomotor regulation of locomotion. Can J Physiol Pharmacol 74(4): 418–425
Simmons AM, Costa LM, Gerstein HB (2004) Lateral line-mediated rheotactic behavior in tadpoles of the African clawed frog (Xenopus laevis). J Comp Physiol A 190(9): 747–758
Sinha SR, Moss CF (2007) Vocal premotor activity in the superior colliculus. J Neurosci 27(1): 98–110
Sperry R (1950) Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol 43: 482–489
Stehouwer DJ (1987) Compensatory eye movements produced during fictive swimming of a deafferented, reduced preparation in vitro. Brain Res 410(2): 264–268 doi:10.1016/0006-8993(87)90323-4
Straka H, Dieringer N (2004) Basic organization principles of the VOR: lessons from frogs. Prog Neurobiol 73: 259–309
Suga N, Jen PH (1975) Peripheral control of acoustic signals in the auditory system of echolocating bats. J Exp Biol 62(2): 277–311
Udo M, Kamei H, Matsukawa K, Tanaka K (1982) Interlimb coordination in cat locomotion investigated with perturbation. Exp Brain Res 46(3): 438–447. doi:10.1007/bf00238638
von Helmholtz H (1866) Handbuch der Physiologischen Optik. English translation by J. P. C. Southall (1924) for the Optical Society of America.
von Holst E, Mittelstaedt H (1950) Das Reafferenzprinzip. Naturwissenschaften 37: 464–476
Webb B (2004) Neural mechanisms for prediction: do insects have forward models. Trends Neurosci 27: 278–282
Weeg MS, Land BR, Bass AH (2005) Vocal pathways modulate efferent neurons to the inner ear and lateral line. J Neurosci 25(25): 5967–5974
Author information
Authors and Affiliations
Corresponding author
Additional information
This article forms part of a special issue of Biological Cybernetics entitled “Multimodal and Sensorimotor Bionics”.
Rights and permissions
About this article
Cite this article
Chagnaud, B.P., Simmers, J. & Straka, H. Predictability of visual perturbation during locomotion: implications for corrective efference copy signaling. Biol Cybern 106, 669–679 (2012). https://doi.org/10.1007/s00422-012-0528-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-012-0528-0