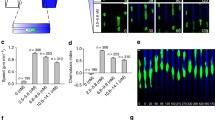
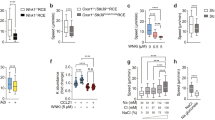
Abstract
Movement toward the source of a chemoattractant gradient is a basic cellular property in health and disease. Enhanced migration during metastasis involves deregulated growth factor signaling. Growth factor stimulation and cell migration converge both on the important second messenger Ca2+. To date, the molecular identification of Ca2+ entry pathways activated by growth factors during chemotaxis is still an open issue. We investigated the involvement of the nonselective Ca2+ channel TRPC1 (transient receptor potential canonical 1) in FGF-2 guided chemotaxis by means of time-lapse video microscopy and by functional Ca2+ measurements. To specifically address TRPC1 function in transformed MDCK cells we altered the expression levels by siRNA or overexpression. We report that TRPC1 channels are required for the orientation of transformed MDCK cells in FGF-2 gradients because TRPC1 knockdown or pharmacological blockade prevented chemotaxis. Stimulation with FGF-2 triggered an immediate Ca2+ influx via TRPC1 channels that depended on phospholipase C and phosphatidylinositol 3-kinase signaling. Impeding this Ca2+ influx abolished chemotaxis toward FGF-2. This functional connection correlated with clustering of FGF receptors and TRPC1 channels as was observed by immunolabeling. These findings show the important interplay between growth factor signaling and Ca2+ influx in chemotaxis.





Similar content being viewed by others
References
Abramowitz J, Birnbaumer L (2009) Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J 23:297–328
Ahmmed GU, Mehta D, Vogel S, Holinstat M, Paria BC, Tiruppathi C, Malik AB (2004) Protein kinase Calpha phosphorylates the TRPC1 channel and regulates store-operated Ca2+ entry in endothelial cells. J Biol Chem 279:20941–20949
Antoniotti S, Lovisolo D, Fiorio Pla A, Munaron L (2002) Expression and functional role of bTRPC1 channels in native endothelial cells. FEBS Lett 510:189–195
Ashish PMS, Perryman PB, Yang L, Yin HL, Krueger JK (2007) Global structure changes associated with Ca2+ activation of full-length human plasma gelsolin. J Biol Chem 282:25884–25892
Bandyopadhyay BC, Swaim WD, Liu X, Redman RS, Patterson RL, Ambudkar IS (2005) Apical localization of a functional TRPC3/TRPC6-Ca2+-signaling complex in polarized epithelial cells. Role in apical Ca2+ influx. J Biol Chem 280:12908–12916
Bowman CL, Gottlieb PA, Suchyna TM, Murphy YK, Sachs F (2007) Mechanosensitive ion channels and the peptide inhibitor GsMTx-4: history, properties, mechanisms and pharmacology. Toxicon 49:249–270
Brazer SC, Singh BB, Liu X, Swaim W, Ambudkar IS (2003) Caveolin-1 contributes to assembly of store-operated Ca2+ influx channels by regulating plasma membrane localization of TRPC1. J Biol Chem 278:27208–27215
Brundage RA, Fogarty KE, Tuft RA, Fay FS (1991) Calcium gradients underlying polarization and chemotaxis of eosinophils. Science 254:703–706
Chen J, Crossland RF, Noorani MMZ, Marrelli SP (2009) Inhibition of TRPC1/TRPC3 by PKG contributes to NO-mediated vasorelaxation. Am J Physiol Heart Circ Physiol 297:H417–H424
Doyle AD, Lee J (2005) Cyclic changes in keratocyte speed and traction stress arise from Ca2+-dependent regulation of cell adhesiveness. J Cell Sci 118:369–379
Dreval V, Dieterich P, Stock C, Schwab A (2005) The role of Ca2+ transport across the plasma membrane for cell migration. Cell Physiol Biochem 16:119–126
Evans JH, Falke JJ (2007) Ca2+ influx is an essential component of the positive-feedback loop that maintains leading-edge structure and activity in macrophages. Proc Natl Acad Sci USA 104:16176–16181
Fabian A, Fortmann T, Dieterich P, Riethmüller C, Schön P, Mally S, Nilius B, Schwab A (2008) TRPC1 channels regulate directionality of migrating cells. Pflugers Arch 457:475–484
Fiorio Pla A, Maric D, Brazer SC, Giacobini P, Liu X, Chang YH, Ambudkar IS, Barker JL (2005) Canonical transient receptor potential 1 plays a role in basic fibroblast growth factor (bFGF)/FGF receptor-1-induced Ca2+ entry and embryonic rat neural stem cell proliferation. J Neurosci 25:2687–2701
Guerrin M, Scotet E, Malecaze F, Houssaint E, Plouet J (1997) Overexpression of vascular endothelial growth factor induces cell transformation in cooperation with fibroblast growth factor 2. Oncogene 14:463–471
Hahn K, DeBiasio R, Taylor DL (1992) Patterns of elevated free calcium and calmodulin activation in living cells. Nature 359:736–738
Haugh JM, Codazzi F, Teruel M, Meyer T (2000) Spatial sensing in fibroblasts mediated by 3′ phosphoinositides. J Cell Biol 151:1269–1280
Hofmann T, Obukhov AG, Schaefe M, Harteneck C, Gudermann T, Schultz G (1999) Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397:259–263
Kessler W, Budde T, Gekle M, Fabian A, Schwab A (2008) Activation of cell migration with fibroblast growth factor-2 requires calcium-sensitive potassium channels. Pflugers Arch 456:813–823
Kölsch V, Charest PG, Firtel RA (2008) The regulation of cell motility and chemotaxis by phospholipid signaling. J Cell Sci 121:551–559
Komuro H, Rakic P (1996) Intracellular Ca2+ fluctuations modulate the rate of neuronal migration. Neuron 17:275–285
Kwabi-Addo B, Ozen M, Ittmann M (2004) The role of fibroblast growth factors and their receptors in prostate cancer. Endocr Relat Cancer 11:709–724
Lee J, Ishihara A, Oxford G, Johnson B, Jacobson K (1999) Regulation of cell movement is mediated by stretch-activated calcium channels. Nature 400:382–386
Lemonnier L, Trebak M, Putney JW Jr (2008) Complex regulation of the TRPC3, 6 and 7 channel subfamily by diacylglycerol and phosphatidylinositol-4, 5-bisphosphate. Cell Calcium 43:506–514
Lintschinger B, Balzer-Geldsetzer M, Baskaran T, Graier WF, Romanin C, Zhu MX, Groschner K (2000) Coassembly of Trp1 and Trp3 proteins generates diacylglycerol- and Ca2+-sensitive cation channels. J Biol Chem 275:27799–27805
Liu X, Bandyopadhyay BC, Singh BB, Groschner K, Ambudkar IS (2005) Molecular analysis of a store-operated and 2-acetyl-sn-glycerol-sensitive non-selective cation channel. Heteromeric assembly of TRPC1-TRPC3. J Biol Chem 280:21600–21606
Louis M, Zanou N, Van Schoor M, Gailly P (2008) TRPC1 regulates skeletal myoblast migration and differentiation. J Cell Sci 121:3951–3959
MacMillan D, McCarron JG (2010) The phospholipase C inhibitor U-73122 inhibits Ca2+ release from the intracellular sarcoplasmic reticulum Ca2+ store by inhibiting Ca2+ pumps in smooth muscle. Br J Pharmacol 160:1295–1301
Merritt JE, Jacob R, Hallam TJ (1989) Use of manganese to discriminate between calcium influx and mobilization from internal stores in stimulated human neutrophils. J Biol Chem 264:1522–1527
Migdal M, Soker S, Yarden Y, Neufeld G (1995) Activation of a transfected FGFR-1 receptor in Madin-Darby epithelial cells results in a reversible loss of epithelial properties. J Cell Physiol 162:266–276
Munaron L, Fiorio Pla A (2000) Calcium influx induced by activation of tyrosine kinase receptors in cultured bovine aortic endothelial cells. J Cell Physiol 185:454–456
Oberleithner H, Westphale HJ, Gassner B (1991) Alkaline stress transforms Madin–Darby canine kidney cells. Pflugers Arch 419:418–420
Saleh SN, Albert AP, Large WA (2009) Activation of native TRPC1/C5/C6 channels by endothelin-1 is mediated by both PIP(3) and PIP(2) in rabbit coronary artery myocytes. J Physiol 587:5361–5375
Saleh SN, Albert AP, Peppiatt-Wildman CM, Large WA (2008) Diverse properties of store-operated TRPC channels activated by protein kinase C in vascular myocytes. J Physiol 586:2463–2476
Schwab A, Finsterwalder F, Kersting U, Danker T, Oberleithner H (1997) Intracellular Ca2+ distribution in migrating transformed epithelial cells. Pflugers Arch 434:70–76
Schwab A, Nechyporuk-Zloy V, Fabian A, Stock C (2007) Cells move when ions and water flow. Pflugers Arch 453:421–432
Shi J, Ju M, Saleh SN, Albert AP, Large WA (2010) TRPC6 channels stimulated by angiotensin II are inhibited by TRPC1/C5 channel activity through a Ca2+- and PKC-dependent mechanism in native vascular myocytes. J Physiol 588:3671–3682
Shim S, Goh EL, Ge S, Sailor K, Yuan JP, Roderick HL, Bootman MD, Worley PF, Song H, Ming GL (2005) XTRPC1-dependent chemotropic guidance of neuronal growth cones. Nat Neurosci 8:730–735
Takeda K, Sasaki AT, Ha H, Seung HA, Firtel RA (2007) Role of phosphatidylinositol 3-kinases in chemotaxis in Dictyostelium. J Biol Chem 282:11874–11884
Trebak M, Lemonnier L, Smyth JT, Vazquez G, Putney Jr JW (2007) Phospholipase C-coupled receptors and activation of TRPC channels. Handb Exp Pharmacol:593–614
Wang F, Herzmark P, Weiner OD, Srinivasan S, Servant G, Bourne HR (2002) Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nat Cell Biol 4:513–518
Wang GX, Poo MM (2005) Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature 434:898–904
Waning J, Vriens J, Owsianik G, Stuwe L, Mally S, Fabian A, Frippiat C, Nilius B, Schwab A (2007) A novel function of capsaicin-sensitive TRPV1 channels: involvement in cell migration. Cell Calcium 42:17–25
Wei C, Wang X, Chen M, Ouyang K, Song LS, Cheng H (2009) Calcium flickers steer cell migration. Nature 457:901–905
Xu HT, Yuan XB, Guan CB, Duan S, Wu CP, Feng L (2004) Calcium signaling in chemorepellant Slit2-dependent regulation of neuronal migration. Proc Natl Acad Sci USA 101:4296–4301
Yang S, Zhang JJ, Huang XY (2009) Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell 15:124–134
Acknowledgement
This work was supported by grants from the Rolf-Dierichs-Stiftung (Medical Faculty, University Münster) (grant number: 193423) and from the Boehringer Ingelheim Fonds to A.F.. A.S. was supported by the IZKF Münster (grant number: Schw 2/030/08) and by the Deutsche Forschungsgemeinschaft (grant number: Schw 407/9-3).
Ethical approval
All experiments were performed in accordance with the ethical standards required by Pflügers Archiv–European Journal of Physiology.
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Anke Fabian and Thomas Fortmann contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Expression of TRPC3 channels
We determined TRPC3 expression in all four cell lines employed in our study: hTRPC1-HA overexpressing cells and the corresponding control cells transfected with an “empty” pcDNA3 expression vector as well as siTRPC1 cells and the corresponding control cells transfected with the pSUPER.retro.puro vector containing a mock oligo. The results of these experiments are summarized in fig. S1.
Western blot analysis of TRPC3 expression in MDCK-F cells with modified TRPC1 expression and in the respective control cell lines. The TRPC3 antibody (alomone) detects a single band of the expected size. Loading of equal amounts of protein was verified by normalizing TRPC3 expression to that of β-actin (fig. S1A). Fig. S1B shows the statistical analysis of four experiments. TRPC3 expression is identical in all four cell lines used in this study.
Knock-down of TRPC3 channels
TRPC3 expression was transiently knocked-down with siRNA technology (Qiagen, Germany) by using the following targeting sequences:
TRPC3_1 (sense, GCUUGUAUUCAAUGCCUCAUU; antisense, UGAGGCAUUGAAUACAAGCAG), TRPC3_2 (sense, CGUGGACAGUUAUGUCCAAUU; antisense, UUGGACAUAACUGUCCACGUA). As a control, a scramled siRNA (sense, AGAUCCGUAUAGUGUACCUUA; antisense, UAAGGUACACUAUACGGAUCU, synthesized by Dharmacon, USA) was used.
siTRPC1-MCDK-F cells were transiently transfected using the transfection reagent Fugene® 6 (Roche, Germany). To this end, cells were grown in a 60mm dish at 80% confluency. Transfection was performed using 3 µl of the transfection reagent with 25nM or 100nM siRNA. After 24 hours the transfection medium was removed and the cells were incubated with serum-free medium for another 24 hours.
48 h post transfection cells were pretreated with 10 ng/ml FGF-2 for 3 h and either used for Western blot analysis of TRPC3 expression or for measurements of the intracellular Ca2+ concentration according to the protocols described in the Methods section.
Reducing TRPC3 expression by 50 – 60 % has no effect on [Ca2+]i of siTRPC1-MDCK-F cells when compared to cells treated with scrambled control siRNA. (PPT 145 kb)
Rights and permissions
About this article
Cite this article
Fabian, A., Fortmann, T., Bulk, E. et al. Chemotaxis of MDCK-F cells toward fibroblast growth factor-2 depends on transient receptor potential canonical channel 1. Pflugers Arch - Eur J Physiol 461, 295–306 (2011). https://doi.org/10.1007/s00424-010-0901-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-010-0901-6