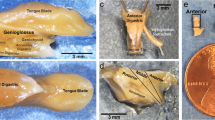
Abstract
Relatively little is known about the underlying neuropathology of dysphagia in amyotrophic lateral sclerosis (ALS); thus, effective treatments remain elusive. Tremendous progress toward understanding and treating dysphagia in ALS may be possible through the use of an animal model of dysphagia in ALS research; however, no such animal model currently exists. The most logical candidate to consider is the SOD1-G93A transgenic mouse, the most widely investigated animal model of ALS. To investigate whether this animal model develops dysphagia, oral behaviors (lick and mastication rates) of SOD1-G93A transgenic mice (n = 30) were evaluated at three time points based on hind limb motor function: asymptomatic (60 days), disease onset (~110 days), and disease end-stage (~140 days). Age-matched nontransgenic littermates (n = 30) served as controls. At each time point, lick and mastication rates were significantly lower (p < 0.05) for transgenic mice compared with controls. Histologic analysis of the brainstem showed marked neurodegeneration (vacuolation) of the trigeminal and hypoglossal nuclei, two key motor components involved in mastication and licking behaviors. These results demonstrate a clinicopathologic correlation of oral dysfunction in SOD1-G93A transgenic mice, thereby establishing the SOD1-G93A transgenic mouse as a bona fide animal model of oral dysphagia in ALS.









Similar content being viewed by others
References
Brownell B, Oppenheimer DR, Hughes JT. The central nervous system in motor neurone disease. J Neurol Neurosurg Psychiatry. 1970;33:338–57.
Charles T, Swash M. Amyotrophic lateral sclerosis: current understanding. J Neurosci Nurs. 2001;33:245–53.
Hughes JT, Jerrome D. Ultrastructure of anterior horn motor neurones in the Hirano-Kurland-Sayre type of combined neurological system degeneration. J Neurol Sci. 1971;13:389–99. doi:10.1016/0022-510X(71)90002-5.
Lawyer T Jr, Netsky MG. Amyotrophic lateral sclerosis. AMA Arch Neurol Psychiatry. 1953;69:171–92.
Rowland LP. Ten central themes in a decade of amyotrophic lateral sclerosis research. New York: Raven Press; 1991.
Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–700. doi:10.1056/NEJM200105313442207.
Talbot K. Motor neurone disease. Postgrad Med J. 2002;78:513–9. doi:10.1136/pmj.78.923.513.
Tandan R, Bradley WG. Amyotrophic lateral sclerosis: Part 1. Clinical features, pathology, and ethical issues in management. Ann Neurol. 1985;18:271–80. doi:10.1002/ana.410180302.
Walling AD. Amyotrophic lateral sclerosis: Lou Gehrig’s disease. Am Fam Physician. 1999;59:1489–96.
Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264:1772–5. doi:10.1126/science.8209258.
Gurney ME. Transgenic animal models of familial amyotrophic lateral sclerosis. J Neurol. 1997;244(Suppl 2):S15–20.
Gurney ME. The use of transgenic mouse models of amyotrophic lateral sclerosis in preclinical drug studies. J Neurol Sci. 1997;152(Suppl 1):S67–73. doi:10.1016/S0022-510X(97)00247-5.
Ripps ME, Huntley GW, Hof PR, Morrison JH, Gordon JW. Transgenic mice expressing an altered murine superoxide dismutase gene provide an animal model of amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 1995;92:689–93. doi:10.1073/pnas.92.3.689.
Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, et al. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–16. doi:10.1016/0896-6273(95)90259-7.
Raoul C, Abbas-Terki T, Bensadoun JC, Guillot S, Haase G, Szulc J, et al. Lentiviral-mediated silencing of SOD1 through RNA interference retards disease onset and progression in a mouse model of ALS. Nat Med. 2005;11:423–8. doi:10.1038/nm1207.
Sasaki S, Warita H, Abe K, Iwata M. Impairment of axonal transport in the axon hillock and the initial segment of anterior horn neurons in transgenic mice with a G93A mutant SOD1 gene. Acta Neuropathol. 2005;110:48–56. doi:10.1007/s00401-005-1021-9.
Zald DH, Pardo JV, The functional neuroanatomy of voluntary swallowing. Ann Neurol. 1999; 46:281–6. doi :10.1002/1531-8249(199909)46:3<281::AID-ANA2>3.0.CO;2-L.
Weydt P, Hong SY, Kliot M, Moller T. Assessing disease onset and progression in the SOD1 mouse model of ALS. Neuroreport. 2003;14:1051–4. doi:10.1097/00001756-200305230-00029.
Newbery HJ, Abbott CM. Of mice, men and motor neurons. Trends Genet. 2001;17:S2–6. doi:10.1016/S0168-9525(01)02459-3.
Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, et al. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS). Proc Natl Acad Sci USA. 2002;99:1604–9. doi:10.1073/pnas.032539299.
Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–49. doi:10.1146/annurev.neuro.27.070203.144244.
Ralph GS, Radcliffe PA, Day DM, Carthy JM, Leroux MA, Lee DC, et al. Silencing mutant SOD1 using RNAi protects against neurodegeneration and extends survival in an ALS model. Nat Med. 2005;11:429–33. doi:10.1038/nm1205.
Higo R, Tayama N, Watanabe T, Nitou T. Videomanofluorometric study in amyotrophic lateral sclerosis. Laryngoscope. 2002;112:911–7. doi:10.1097/00005537-200205000-00024.
Kawai S, Tsukuda M, Mochimatsu I, Enomoto H, Kagesato Y, Hirose H, et al. A study of the early stage of dysphagia in amyotrophic lateral sclerosis. Dysphagia. 2003;18:1–8. doi:10.1007/s00455-002-0074-3.
Hillel AD, Miller RM. Management of bulbar symptoms in amyotrophic lateral sclerosis. Adv Exp Med Biol. 1987;209:201–21.
Tayama N. Dysphagia in amyotrophic lateral sclerosis–the mechanism and managements. Rinsho Shinkeigaku. 1995;35:1557–9.
Ohkubo H. Dysphagia in amyotrophic lateral sclerosis–electromyographic and radiological investigations. Otol Fukuoka. 1980;26:44–78.
Hillel AD, Miller R. Bulbar amyotrophic lateral sclerosis: patterns of progression and clinical management. Head Neck. 1989;11:51–9. doi:10.1002/hed.2880110110.
Zang DW, Yang Q, Wang HX, Egan G, Lopes EC, Cheema SS. Magnetic resonance imaging reveals neuronal degeneration in the brainstem of the superoxide dismutase 1 transgenic mouse model of amyotrophic lateral sclerosis. Eur J Neurosci. 2004;20:1745–51. doi:10.1111/j.1460-9568.2004.03648.x.
Angenstein F, Niessen HG, Goldschmidt J, Vielhaber S, Ludolph AC, Scheich H. Age-dependent changes in MRI of motor brain stem nuclei in a mouse model of ALS. Neuroreport. 2004;15:2271–4. doi:10.1097/00001756-200410050-00026.
Gannon KS, Smith JC, Henderson R, Hendrick P. A system for studying the microstructure of ingestive behavior in mice. Physiol Behav. 1992;51:515–21. doi:10.1016/0031-9384(92)90173-Y.
Kobayashi M, Masuda Y, Fujimoto Y, Matsuya T, Yamamura K, Yamada Y, et al. Electrophysiological analysis of rhythmic jaw movements in the freely moving mouse. Physiol Behav. 2002;75:377–85. doi:10.1016/S0031-9384(01)00662-X.
Okayasu I, Yamada Y, Kohno S, Yoshida N. New animal model for studying mastication in oral motor disorders. J Dent Res. 2003;82:318–21.
Koga Y, Yoshida N, Kobayashi K, Ichiro O, Yamada Y. Development of a three-dimensional jaw-tracking system implanted in the freely moving mouse. Med Eng Phys. 2001;23:201–6. doi:10.1016/S1350-4533(01)00038-8.
Carvalho TC, Gerstner GE. Licking rate adaptations to increased mandibular weight in the adult rat. Physiol Behav. 2004;82:331–7. doi:10.1016/j.physbeh.2004.04.003.
Strand EA, Miller RM, Yorkston KM, Hillel AD. Management of oral-pharyngeal dysphagia symptoms in amyotrophic lateral sclerosis. Dysphagia. 1996;11:129–39. doi:10.1007/BF00417903.
Genotyping Protocol for SOD. Bar Harbor, ME: The Jackson Laboratory, 2005.
Heiman-Patterson TD, Deitch JS, Blankenhorn EP, Erwin KL, Perreault MJ, Alexander BK, et al. Background and gender effects on survival in the TgN(SOD1–G93A)1Gur mouse model of ALS. J Neurol Sci. 2005;236:1–7. doi:10.1016/j.jns.2005.02.006.
Xu Z, Jung C, Higgins C, Levine J, Kong J. Mitochondrial degeneration in amyotrophic lateral sclerosis. J Bioenerg Biomembr. 2004;36:395–9. doi:10.1023/B:JOBB.0000041774.12654.e1.
Kong J, Xu Z. Massive mitochondrial degeneration in motor neurons triggers the onset of amyotrophic lateral sclerosis in mice expressing a mutant SOD1. J Neurosci. 1998;18:3241–50.
Miana-Mena FJ, Munoz MJ, Yague G, Mendez M, Moreno M, Ciriza J, et al. Optimal methods to characterize the G93A mouse model of ALS. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6:55–62. doi:10.1080/17434470510045230.
Hamadeh MJ, Rodriguez MC, Kaczor JJ, Tarnopolsky MA. Caloric restriction transiently improves motor performance but hastens clinical onset of disease in the Cu/Zn-superoxide dismutase mutant G93A mouse. Muscle Nerve. 2005;31:214–20. doi:10.1002/mus.20255.
Barneoud P, Curet O. Beneficial effects of lysine acetylsalicylate, a soluble salt of aspirin, on motor performance in a transgenic model of amyotrophic lateral sclerosis. Exp Neurol. 1999;155:243–51. doi:10.1006/exnr.1998.6984.
Weijnen JA. Licking behavior in the rat: measurement and situational control of licking frequency. Neurosci Biobehav Rev. 1998;22:751–60. doi:10.1016/S0149-7634(98)00003-7.
Hiiemae KM, Palmer JB. Food transport and bolus formation during complete feeding sequences on foods of different initial consistency. Dysphagia. 1999;14:31–42. doi:10.1007/PL00009582.
Silani V, Kasarskis EJ, Yanagisawa N. Nutritional management in amyotrophic lateral sclerosis: a worldwide perspective. J Neurol. 1998;245(Suppl 2):S13–9. discussion S29.
Hillel A, Dray T, Miller R, Yorkston K, Konikow N, Strande E, et al. Presentation of ALS to the otolaryngologist/head and neck surgeon: getting to the neurologist. Neurology. 1999;53:S22–5. discussion S35–S26.
Haenggeli C, Kato AC. Differential vulnerability of cranial motoneurons in mouse models with motor neuron degeneration. Neurosci Lett. 2002;335:39–43. doi:10.1016/S0304-3940(02)01140-0.
Allen TC. AFIP laboratory methods in histotechnology. Washington, DC: American Registry of Pathology; 1992.
Bucher S, Braunstein KE, Niessen HG, Kaulisch T, Neumaier M, Boeckers TM, et al. Vacuolization correlates with spin-spin relaxation time in motor brainstem nuclei and behavioural tests in the transgenic G93A-SOD1 mouse model of ALS. Eur J Neurosci. 2007;26:1895–901. doi:10.1111/j.1460-9568.2007.05831.x.
Paxinos G, Franklin K. The mouse brain in sterotaxic coordinates. 2nd ed. Sydney, Australia: Academic Press; 2001.
Little RJA, Rubin DB. Statistical analysis with missing data. 2nd ed. Hoboken, NJ: John Wiley & Sons, Inc.; 2002.
Singer J. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat. 1998;24:323–55.
Peugh J, Enders C. Using the SPSS mixed procedure to fit cross-sectional and longitudinal multilevel models. Educ Psychol Meas. 2005;65:717–41. doi:10.1177/0013164405278558.
Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS systems for mixed models. Cary, NC: SAS Institute, Inc.; 1996.
Forthofer RN, Lee ES, Hernandez M. Biostatistics: a guide to design, analysis, and discovery. 2nd ed. Burlington, MA: Elsevier; 2007.
Veldink JH, Bar PR, Joosten EA, Otten M, Wokke JH, van den Berg LH. Sexual differences in onset of disease and response to exercise in a transgenic model of ALS. Neuromuscul Disord. 2003;13:737–43. doi:10.1016/S0960-8966(03)00104-4.
Lowry KS, Murray SS, McLean CA, Talman P, Mathers S, Lopes EC, et al. A potential role for the p75 low-affinity neurotrophin receptor in spinal motor neuron degeneration in murine and human amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2001;2:127–34. doi:10.1080/146608201753275463.
Azari MF, Lopes EC, Stubna C, Turner BJ, Zang D, Nicola NA, et al. Behavioural and anatomical effects of systemically administered leukemia inhibitory factor in the SOD1(G93A G1H) mouse model of familial amyotrophic lateral sclerosis. Brain Res. 2003;982:92–7. doi:10.1016/S0006-8993(03)02989-5.
Turner BJ, Cheah IK, Macfarlane KJ, Lopes EC, Petratos S, Langford SJ, et al. Antisense peptide nucleic acid-mediated knockdown of the p75 neurotrophin receptor delays motor neuron disease in mutant SOD1 transgenic mice. J Neurochem. 2003;87:752–63. doi:10.1046/j.1471-4159.2003.02053.x.
Druzinsky RE. The time allometry of mammalian chewing movements: chewing frequency scales with body mass in mammals. J Theor Biol. 1993;160:427–40. doi:10.1006/jtbi.1993.1028.
Kasarskis EJ, Berryman S, Vanderleest JG, Schneider AR, McClain CJ. Nutritional status of patients with amyotrophic lateral sclerosis: relation to the proximity of death. Am J Clin Nutr. 1996;63:130–7.
Dal Canto MC, Gurney ME. Neuropathological changes in two lines of mice carrying a transgene for mutant human Cu, Zn SOD, and in mice overexpressing wild type human SOD: a model of familial amyotrophic lateral sclerosis (FALS). Brain Res. 1995;676:25–40. doi:10.1016/0006-8993(95)00063-V.
Acknowledgments
The authors gratefully acknowledge Elena Pak, Tiepha Renee Cooper, Cory Morris, Sameer Dongre, Emmanuelle Simon, Waseem Ahmed, Emily Barrett, Vladim Bobrovnikov, Mohamed Raafat, and Di Wu for their invaluable assistance with data collection. We also express our gratitude to Ms. Joani Zary and Dr. Hubert Burden from the Department of Anatomy and Cell Biology at East Carolina University for their expert guidance in histologic methods. We also are grateful to the veterinarians and animal technicians in the Department of Comparative Medicine at East Carolina University who kindly maintained the mouse colony for this investigation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lever, T.E., Gorsek, A., Cox, K.T. et al. An Animal Model of Oral Dysphagia in Amyotrophic Lateral Sclerosis. Dysphagia 24, 180–195 (2009). https://doi.org/10.1007/s00455-008-9190-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-008-9190-z