Abstract
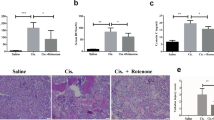
Necroptosis is a nonapoptotic cell death pathway. We aim to study the effect of necrostatin-1 (a specific necroptosis inhibitor) in cisplatin-induced injury. We analyzed the effect of the combined use of inhibitors of apoptosis (z-vad) and necroptosis (necrostatin-1) in acute kidney injury by cisplatin in human proximal tubule cells. Our results showed moderate effectiveness in cytoprotection after treatment with z-vad. But the concomitant use of inhibitors (z-vad and necrostatin-1) presented synergistic and additive protection. The present study analyzed the caspase-3 activity and we observed a significant decrease in the group treated with z-vad and cisplatin. However we did not observe changes in the group treated with both inhibitors (z-vad and necrostatin-1) and cisplatin. Thus, demonstrating that necroptosis is a caspase-independent mechanism. We also analyzed the effect of necrostatin-1 in vivo model. C57BL/6 mice were treated with cisplatin and/or inhibitors. The concomitant use of inhibitors (z-vad and necrostatin-1) recovered renal function and decreased levels of urinary Ngal. Additionally, we analyzed the expression of RIP-1, a specific marker for necroptosis. In animals treated with cisplatin and z-VAD levels of RIP-1 were higher. This result reinforces that necroptosis occurs only in conditions where apoptosis was blocked. However, the use of both inhibitors (z-vad and necrostatin-1) provided additional protection. In conclusion, our study has a significant potential to show in vitro and in vivo protection obtained by necrostatin-1. Therefore, our results suggest that necroptosis may be an important mechanism of cell death after kidney injury.



Similar content being viewed by others
References
Case J, Khan S, Khalid R, Khan A (2013) Epidemiology of acute kidney injury in the intensive care unit. Crit Care Res Pract 2013:1–9
Han WK (2012) Biomarkers for early detection of acute kidney injury. Curr Biom Find 2:77–85
Palevsky PM (2013) Renal replacement therapy in acute kidney injury. Adv Chronic Kidney Dis 20(1):76–84
Prowle JR (2013) Acute kidney injury: an intensivist’s perspective. Pediatr Nephrol 29(1):13–21
Ibrahim AE, Sarhane KA, Fagan SP, Goverman J (2013) Renal dysfunction in burns: a review. Ann Burns Fire Disasters 26(1):16–25
Kam Tao Li P, Burdmann EA, Mehta RL (2013) Acute kidney injury: global health alert. J Nephropathol 2(2):90–97
Vanmassenhove J, Vanholder R, Nagler E, Van Biesen W (2013) Urinary and serum biomarkers for the diagnosis of acute kidney injury: an in-depth review of the literature. Nephrol Dial Transplant 28(2):254–273
Linkermann A, De Zen F, Weinberg J, Kunzendorf U, Krautwald S (2012) Programmed necrosis in acute kidney injury. Nephrol Dial Transplant 27(9):3412–3419
Wei Q, Dong G, Yang T, Megyesi J, Price PM et al (2007) Activation and involvement of p53 in cisplatin-induced nephrotoxicity. Am J Physiol Renal Physiol 293(4):1282–1291
Antczak C, Takagi T, Ramirez CN, Radu C, Djaballah H (2009) Live-cell imaging of caspase activation for high-content screening. J Biomol Screen 14(8):956–969
Degterev A, Huang Z, Boyce M, Li Y, Jagtap P et al (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1(2):112–119
Tristão VR, Gonçalves PF, Dalboni MA, Batista MC, de Durão MS Jr et al (2012) Nec-1 protects against nonapoptotic cell death in cisplatin-induced kidney injury. Ren Fail 34(3):373–377
Smith CC, Davidson SM, Lim SY, Simpkin JC, Hothersall JS et al (2007) Necrostatin: a potentially novel cardioprotective agent? Cardiovasc Drugs Ther 21(4):227–233
Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES et al (2009) Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 16:3–11
Li Y, Yang X, Ma C, Qiao J, Zhang C (2008) Necroptosis contributes to the NMDA-induced excitotoxicity in rat’s cultured cortical neurons. Neurosci Lett 447(2–3):120–123
Han W, Xie J, Li L, Liu Z, Hu X (2009) Necrostatin-1 reverts shikonin-induced necroptosis to apoptosis. Apoptosis 14(5):674–686
Daemen MA, van’t Veer C, Denecker G, Heemskerk VH, Wolfs TG et al (1999) Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J Clin Invest 104(5):541–549
Guo R, Wang Y, Minto AW, Quigg RJ, Cunningham PN (2004) Acute renal failure in endotoxemia is dependent on caspase activation. J Am Soc Nephrol 15(12):3093–3102
Cummings BS, Schnellmann RG (2002) Cisplatin-induced renal cell apoptosis: caspase 3-dependent and -independent pathways. J Pharmacol Exp Ther 302(1):8–17
Xu X, Chua KW, Chua CC, Liu CF, Hamdy RC et al (2010) Synergistic protective effects of humanin and necrostatin-1 on hypoxia and ischemia/reperfusion injury. Brain Res 1355:189–194
Sawicka E, Długosz A, Rembacz KP, Guzik A (2013) The effects of coenzyme Q10 and baicalin in cisplatin-induced lipid peroxidation and nitrosative stress. Acta Pol Pharm 70(6):977–985
Tiwari MM, Brock RW, Megyesi JK, Kaushal GP, Mayeux PR (2005) Disruption of renal peritubular blood flow in lipopolysaccharide-induced renal failure: role of nitric oxide and caspases. Am J Physiol Renal Physiol 289(6):1324–1332
Linkermann A, Bräsen JH, Himmerkus N, Liu S, Huber TB et al (2012) Rip-1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int 81(8):751–761
Li J, Wang Y, Du L, Xu C, Cao J et al (2014) Radiation-induced cytochrome c release and the neuroprotective effects of the pan-caspase inhibitor z-VAD-fmk in the hypoglossal nucleus. Exp Ther Med 7(2):383–388
Linkermann A, Bräsen JH, Darding M, Jin MK, Sanz AB et al (2013) Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci USA 110(29):12024–12029
Price PM, Hodeify R (2012) A possible mechanism of renal cell death after ischemia/reperfusion. Kidney Int 81(8):720–721
Haase-Fielitz A, Bellomo R, Devarajan P, Bennett M, Story D (2009) The predictive performance of plasma neutrophil gelatinase-associated lipocalin (NGAL) increases with grade of acute kidney injury. Nephrol Dial Transplant 24:3349–3354
Tonomura Y, Tsuchiya N, Torii M, Uehara T (2010) Evaluation of the usefulness of urinary biomarkers for nephrotoxicity in rats. Toxicology 273(1–3):53–59
Mori K, Nakao K (2007) Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int 71(10):967–970
Mishra J, Mori K, Ma Q, Kelly C, Yang J et al (2004) Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 15(12):3073–3082
Quesada A, Vargas F, Montoro-Molina S, O’Valle F, Rodríguez-Martínez MD et al (2012) Urinary aminopeptidase activities as early and predictive biomarkers of renal dysfunction in cisplatin-treated rats. PLoS One 7(7):40402
Zarjou A, Bolisetty S, Joseph R, Traylor A, Apostolov EO et al (2013) Proximal tubule H-ferritin mediates iron trafficking in acute kidney injury. J Clin Invest 123(10):4423–4434
Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV (2004) Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol 286(3):552–563
Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV (2006) Urinary kidney injury molecule-1: sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol 290(2):517–529
Bonventre JV (2009) Kidney injury molecule-1 (KIM-1): a urinary biomarker and much more. Nephrol Dial Transplant 24(11):3265–3268
Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K et al (2003) Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14(10):2534–2543
Bonventre JV (2003) Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol 1:55–61
Al-Kharusi N, Babiker HA, Al-Salam S, Waly MI, Nemmar A et al (2013) Ellagic acid protects against cisplatin-induced nephrotoxicity in rats: a dose-dependent study. Eur Rev Med Pharmacol Sci 17(3):299–310
Acknowledgments
This study was funded by Grants from Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) No. 08/09773-4, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Tristão, V.R., Pessoa, E.A., Nakamichi, R. et al. Synergistic effect of apoptosis and necroptosis inhibitors in cisplatin-induced nephrotoxicity. Apoptosis 21, 51–59 (2016). https://doi.org/10.1007/s10495-015-1190-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-015-1190-5