Abstract
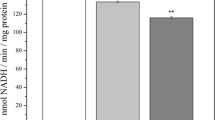
Oxidative stress and mitochondrial damage are implicated in the evolution of neurodegenerative diseases. Increased oxidative damage in specific brain regions during aging might render the brain susceptible to degeneration. Previously, we demonstrated increased oxidative damage and lowered antioxidant function in substantia nigra during aging making it vulnerable to degeneration associated with Parkinson’s disease. To understand whether aging contributes to the vulnerability of brain regions in Alzheimer’s disease, we assessed the oxidant and antioxidant markers, glutathione (GSH) metabolic enzymes, glial fibrillary acidic protein (GFAP) expression and mitochondrial complex I (CI) activity in hippocampus (HC) and frontal cortex (FC) compared with cerebellum (CB) in human brains with increasing age (0.01–80 years). We observed significant increase in protein oxidation (HC: p = 0.01; FC: p = 0.0002) and protein nitration (HC: p = 0.001; FC: p = 0.02) and increased GFAP expression (HC: p = 0.03; FC: p = 0.001) with a decreasing trend in CI activity in HC and FC compared to CB with increasing age. These changes were associated with a decrease in antioxidant enzyme activities, such as superoxide dismutase (HC: p = 0.005), catalase (HC: p = 0.02), thioredoxin reductase (FC: p = 0.04), GSH reductase (GR) (HC: p = 0.005), glutathione-s-transferase (HC: p = 0.0001; FC: p = 0.03) and GSH (HC: p = 0.01) with age. However, these parameters were relatively unaltered in CB. We suggest that the regions HC and FC are subjected to widespread oxidative stress, loss of antioxidant function and enhanced GFAP expression during aging which might make them more susceptible to deranged physiology and selective neuronal degeneration.





Similar content being viewed by others
Abbreviations
- HC:
-
Hippocampus
- FC:
-
Frontal cortex
- CB:
-
Cerebellum
- AD:
-
Alzheimer’s disease
- PMI:
-
Postmortem interval
- MCI:
-
Mild cognitive impairment
- SOD:
-
Superoxide dismutase
- GSH:
-
Glutathione
- GR:
-
Glutathione reductase
- GPx:
-
Glutathione peroxidase
- GST:
-
Glutathione-s-transferase
- GFAP:
-
Glial fibrillay acidic protein
- GCL:
-
Glutamylcysteine ligase
- ThrR:
-
Thioredoxin reductase
References
Mythri RB, Venkateshappa C, Harish G, Mahadevan A, Muthane UB, Yasha TC, Srinivas Bharath MM, Shankar SK (2011) Evaluation of markers of oxidative stress, antioxidant function and astrocytic proliferation in the striatum and frontal cortex of Parkinson’s disease brains. Neurochem Res 36(8):1452–1463. doi:10.1007/s11064-011-0471-9
Venkateshappa C, Harish G, Mythri RB, Mahadevan A, Srinivas Bharath MM, Shankar SK (2011) Increased oxidative damage and decreased antioxidant function in aging human substantia nigra compared to striatum: implications for Parkinson’s disease. Neurochem Res. doi:10.1007/s11064-011-0619-7
Harman D (2006) Alzheimer’s disease pathogenesis: role of aging. Ann N Y Acad Sci 1067:454–460. doi:10.1196/annals.1354.065
Mandal PK, Tripathi M, Sugunan S (2012) Brain oxidative stress: detection and mapping of anti-oxidant marker ‘Glutathione’ in different brain regions of healthy male/female, MCI and Alzheimer patients using non-invasive magnetic resonance spectroscopy. Biochem Biophys Res Commun 417(1):43–48. doi:10.1016/j.bbrc.2011.11.047
Ansari MA, Scheff SW (2010) Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J Neuropathol Exp Neurol 69(2):155–167. doi:10.1097/NEN.0b013e3181cb5af4
Pocernich CB, Butterfield DA (2011) Elevation of glutathione as a therapeutic strategy in Alzheimer disease. Biochim Biophys Acta. doi:10.1016/j.bbadis.2011.10.003
Pamplona R, Dalfo E, Ayala V, Bellmunt MJ, Prat J, Ferrer I, Portero-Otin M (2005) Proteins in human brain cortex are modified by oxidation, glycoxidation, and lipoxidation. Effects of Alzheimer disease and identification of lipoxidation targets. J Biol Chem 280(22):21522–21530. doi:10.1074/jbc.M502255200
Korolainen MA, Auriola S, Nyman TA, Alafuzoff I, Pirttila T (2005) Proteomic analysis of glial fibrillary acidic protein in Alzheimer’s disease and aging brain. Neurobiol Dis 20(3):858–870. doi:10.1016/j.nbd.2005.05.021
Eikelenboom P, Rozemuller AJ, Hoozemans JJ, Veerhuis R, van Gool WA (2000) Neuroinflammation and Alzheimer disease: clinical and therapeutic implications. Alzheimer Dis Assoc Disord 14(Suppl 1):S54–S61
Harish G, Venkateshappa C, Mahadevan A, Pruthi N, Bharath MMS, Shankar SK (2011) Effect of storage time, postmortem interval, agonal state, and gender on the postmortem preservation of glial fibrillary acidic protein and oxidatively damaged proteins in human brains. Biopreserv Biobank. doi:10.1089/bio.2011.0033
Harish G, Venkateshappa C, Mahadevan A, Pruthi N, Srinivas Bharath MM, Shankar SK (2011) Glutathione metabolism is modulated by postmortem interval, gender difference and agonal state in postmortem human brains. Neurochem Int. doi:10.1016/j.neuint.2011.08.024
Chandana R, Mythri RB, Mahadevan A, Shankar SK, Srinivas Bharath MM (2009) Biochemical analysis of protein stability in human brain collected at different post-mortem intervals. Indian J Med Res 129(2):189–199
Jagatha B, Mythri RB, Vali S, Bharath MM (2008) Curcumin treatment alleviates the effects of glutathione depletion in vitro and in vivo: therapeutic implications for Parkinson’s disease explained via in silico studies. Free Radic Biol Med 44(5):907–917. doi:10.1016/j.freeradbiomed.2007.11.011
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Frasca JM, Parks VR (1965) A routine techbique for double staining ultrathin sections using uranyl and lead salts. J Cell Biol 25:157–161
Mythri RB, Jagatha B, Pradhan N, Andersen J, Bharath MM (2007) Mitochondrial complex I inhibition in Parkinson’s disease: how can curcumin protect mitochondria? Antioxid Redox Signal 9(3):399–408. doi:10.1089/ars.2007.9.ft-25
Trounce IA, Kim YL, Jun AS, Wallace DC (1996) Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol 264:484–509
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Bagnyukova TV, Storey KB, Lushchak VI (2003) Induction of oxidative stress in Rana ridibunda during recovery from winter hibernation. J Therm Biol 28:21–28
Guthenberg C, Alin P, Mannervik B (1985) Glutathione transferase from rat testis. Methods Enzymol 113:507–510
Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113:484–490
Flohe L, Gunzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–121
Tietze F (1969) Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 27(3):502–522
Seelig GF, Meister A (1985) Glutathione biosynthesis; gamma-glutamylcysteine synthetase from rat kidney. Methods Enzymol 113:379–390
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443(7113):787–795. doi:10.1038/nature05292
Mao P (1812) Reddy PH (2011) Aging and amyloid beta-induced oxidative DNA damage and mitochondrial dysfunction in Alzheimer’s disease: implications for early intervention and therapeutics. Biochim Biophys Acta 11:1359–1370. doi:10.1016/j.bbadis.2011.08.005
Muller WE, Eckert A, Kurz C, Eckert GP, Leuner K (2010) Mitochondrial dysfunction: common final pathway in brain aging and Alzheimer’s disease–therapeutic aspects. Mol Neurobiol 41(2–3):159–171. doi:10.1007/s12035-010-8141-5
Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G (1997) Widespread peroxynitrite-mediated damage in Alzheimer’s disease. J Neurosci 17(8):2653–2657
Lenaz G, Bovina C, Castelluccio C, Fato R, Formiggini G, Genova ML, Marchetti M, Pich MM, Pallotti F, Parenti Castelli G, Biagini G (1997) Mitochondrial complex I defects in aging. Mol Cell Biochem 174(1–2):329–333
Davey GP, Peuchen S, Clark JB (1998) Energy thresholds in brain mitochondria. Potential involvement in neurodegeneration. J Biol Chem 273(21):12753–12757
Lee YJ, Han SB, Nam SY, Oh KW, Hong JT (2010) Inflammation and Alzheimer’s disease. Arch Pharm Res 33(10):1539–1556. doi:10.1007/s12272-010-1006-7
Liu H, Wang H, Shenvi S, Hagen TM, Liu RM (2004) Glutathione metabolism during aging and in Alzheimer disease. Ann N Y Acad Sci 1019:346–349. doi:10.1196/annals.1297.059
Bonda DJ, Wang X, Perry G, Nunomura A, Tabaton M, Zhu X, Smith MA (2010) Oxidative stress in Alzheimer disease: a possibility for prevention. Neuropharmacology 59(4–5):290–294. doi:10.1016/j.neuropharm.2010.04.005
Butterfield DA, Poon HF, St Clair D, Keller JN, Pierce WM, Klein JB, Markesbery WR (2006) Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: insights into the development of Alzheimer’s disease. Neurobiol Dis 22(2):223–232. doi:10.1016/j.nbd.2005.11.002
Sultana R, Poon HF, Cai J, Pierce WM, Merchant M, Klein JB, Markesbery WR, Butterfield DA (2006) Identification of nitrated proteins in Alzheimer’s disease brain using a redox proteomics approach. Neurobiol Dis 22(1):76–87. doi:10.1016/j.nbd.2005.10.004
Butterfield DA, Reed TT, Perluigi M, De Marco C, Coccia R, Keller JN, Markesbery WR, Sultana R (2007) Elevated levels of 3-nitrotyrosine in brain from subjects with amnestic mild cognitive impairment: implications for the role of nitration in the progression of Alzheimer’s disease. Brain Res 1148:243–248. doi:10.1016/j.brainres.2007.02.084
Lovell MA, Ehmann WD, Butler SM, Markesbery WR (1995) Elevated thiobarbituric acid-reactive substances and antioxidant enzyme activity in the brain in Alzheimer’s disease. Neurology 45(8):1594–1601
Padurariu M, Ciobica A, Hritcu L, Stoica B, Bild W, Stefanescu C (2010) Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer’s disease. Neurosci Lett 469(1):6–10. doi:10.1016/j.neulet.2009.11.033
Reed TT, Pierce WM, Markesbery WR, Butterfield DA (2009) Proteomic identification of HNE-bound proteins in early Alzheimer disease: Insights into the role of lipid peroxidation in the progression of AD. Brain Res 1274:66–76. doi:10.1016/j.brainres.2009.04.009
Sultana R, Piroddi M, Galli F, Butterfield DA (2008) Protein levels and activity of some antioxidant enzymes in hippocampus of subjects with amnestic mild cognitive impairment. Neurochem Res 33(12):2540–2546. doi:10.1007/s11064-008-9593-0
Bermejo P, Martin-Aragon S, Benedi J, Susin C, Felici E, Gil P, Ribera JM, Villar AM (2008) Peripheral levels of glutathione and protein oxidation as markers in the development of Alzheimer’s disease from Mild Cognitive Impairment. Free Radic Res 42(2):162–170. doi:10.1080/10715760701861373
Hillered L, Chan PH (1988) Effects of arachidonic acid on respiratory activities in isolated brain mitochondria. J Neurosci Res 19(1):94–100. doi:10.1002/jnr.490190113
Zhang Y, Marcillat O, Giulivi C, Ernster L, Davies KJ (1990) The oxidative inactivation of mitochondrial electron transport chain components and ATPase. J Biol Chem 265(27):16330–16336
Chinta SJ, Andersen JK (2006) Reversible inhibition of mitochondrial complex I activity following chronic dopaminergic glutathione depletion in vitro: implications for Parkinson’s disease. Free Radic Biol Med 41(9):1442–1448. doi:10.1016/j.freeradbiomed.2006.08.002
Murchison D, Griffith WH (2007) Calcium buffering systems and calcium signaling in aged rat basal forebrain neurons. Aging Cell 6(3):297–305. doi:10.1111/j.1474-9726.2007.00293.x
Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD (1990) Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem 54(3):823–827
Schapira AH (2010) Complex I: inhibitors, inhibition and neurodegeneration. Exp Neurol 224(2):331–335. doi:10.1016/j.expneurol.2010.03.028
Colombrita C, Calabrese V, Stella AM, Mattei F, Alkon DL, Scapagnini G (2003) Regional rat brain distribution of heme oxygenase-1 and manganese superoxide dismutase mRNA: relevance of redox homeostasis in the aging processes. Exp Biol Med (Maywood) 228(5):517–524
Ciavardelli D, Silvestri E, Del Viscovo A, Bomba M, De Gregorio D, Moreno M, Di Ilio C, Goglia F, Canzoniero LM, Sensi SL (2010) Alterations of brain and cerebellar proteomes linked to Abeta and tau pathology in a female triple-transgenic murine model of Alzheimer’s disease. Cell Death Dis 1:e90. doi:10.1038/cddis.2010.68
Wu X, Jiang X, Marini AM, Lipsky RH (2005) Delineating and understanding cerebellar neuroprotective pathways: potential implication for protecting the cortex. Ann N Y Acad Sci 1053:39–47. doi:10.1196/annals.1344.004
Khatoon S, Grundke-Iqbal I, Iqbal K (1994) Levels of normal and abnormally phosphorylated tau in different cellular and regional compartments of Alzheimer disease and control brains. FEBS Lett 351(1):80–84
Causevic M, Farooq U, Lovestone S, Killick R (2010) Beta-Amyloid precursor protein and tau protein levels are differently regulated in human cerebellum compared to brain regions vulnerable to Alzheimer’s type neurodegeneration. Neurosci Lett 485(3):162–166. doi:10.1016/j.neulet.2010.08.088
Williams AJ, Wei HH, Dave JR, Tortella FC (2007) Acute and delayed neuroinflammatory response following experimental penetrating ballistic brain injury in the rat. J Neuroinflammation 4:17. doi:10.1186/1742-2094-4-17
Zlotnik I (1968) The reaction of astrocytes to acute virus infections of the central nervous system. Br J Exp Pathol 49(6):555–564
Rozovsky I, Wei M, Morgan TE, Finch CE (2005) Reversible age impairments in neurite outgrowth by manipulations of astrocytic GFAP. Neurobiol Aging 26(5):705–715. doi:10.1016/j.neurobiolaging.2004.06.009
Hayakawa N, Kato H, Araki T (2007) Age-related changes of astorocytes, oligodendrocytes and microglia in the mouse hippocampal CA1 sector. Mech Ageing Dev 128(4):311–316. doi:10.1016/j.mad.2007.01.005
Porchet R, Probst A, Bouras C, Draberova E, Draber P, Riederer BM (2003) Analysis of glial acidic fibrillary protein in the human entorhinal cortex during aging and in Alzheimer’s disease. Proteomics 3(8):1476–1485. doi:10.1002/pmic.200300456
Sandhir R, Onyszchuk G, Berman NE (2008) Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Exp Neurol 213(2):372–380. doi:10.1016/j.expneurol.2008.06.013
Ross GW, O’Callaghan JP, Sharp DS, Petrovitch H, Miller DB, Abbott RD, Nelson J, Launer LJ, Foley DJ, Burchfiel CM, Hardman J, White LR (2003) Quantification of regional glial fibrillary acidic protein levels in Alzheimer’s disease. Acta Neurol Scand 107(5):318–323
Acknowledgments
The authors thank the Human Brain Tissue Repository (HBTR), NIMHANS, Bangalore, India, for providing the human brain tissue samples required for the study. This study was financially supported by a grant from the Indian Council of Medical Research (ICMR IRIS ID No. 2009-07710) (to MMSB). VC gratefully acknowledges the financial support from Sri Siddhartha Medical College, Tumkur, India. GH is supported by a senior research fellowship from ICMR, India. The authors gratefully acknowledge the donors and their relatives for the kind gift of human brains for neurobiological studies.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Venkateshappa, C., Harish, G., Mahadevan, A. et al. Elevated Oxidative Stress and Decreased Antioxidant Function in the Human Hippocampus and Frontal Cortex with Increasing Age: Implications for Neurodegeneration in Alzheimer’s Disease. Neurochem Res 37, 1601–1614 (2012). https://doi.org/10.1007/s11064-012-0755-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0755-8