Abstract
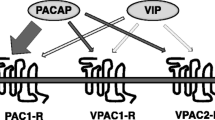
Pituitary adenylate cyclase-activating polypeptide (PACAP) and vasoactive intestinal peptide (VIP) are two closely related peptides, which can activate protein kinase A (PKA). At least three receptors for PACAP and VIP have been identified. The PACAP-specific receptor, PAC1 receptor, exhibits a higher affinity for PACAP than VIP, whereas VIP receptors, VPAC1-R and VPAC2-R, have similar affinities for PACAP and VIP. Both PACAP/VIP and their cognate receptors are highly expressed in the brain, including the hippocampus. Recently, their roles in the regulation of synaptic transmission have begun to emerge. PACAP/VIP can signal through different pathways to regulate N-methyl-d-aspartate (NMDA) receptors in CA1 pyramidal cells. The activation of VPAC1/2-Rs increases evoked NMDA currents via the cyclic AMP/PKA pathway. However, the activation of PAC1-R stimulates a PLC/PKC/Pyk2/Src signaling pathway to enhance NMDA receptor function in hippocampal neurons. Furthermore, different concentrations of PACAP induce different effects on the both α-amino-3-hydroxy-5-isoxazole-propionic acid-evoked current and basal synaptic transmission by activating different receptors. Their roles in learning and memory are also demonstrated using transgenic mice and pharmacological methods.


Similar content being viewed by others
References
Abraham WC (2008) Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci 9:387
Abraham WC, Bear MF (1996) Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci 19:126–130
Arimura A (2007) PACAP: the road to discovery. Peptides 28:1617–1619
Berberich S, Punnakkal P, Jensen V, Pawlak V, Seeburg PH, Hvalby O, Kohr G (2005) Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J Neurosci 25:6907–6910
Ciccarelli E, Vilardaga JP, De NP, Di PE, Waelbroeck M, Bollen A, Robberecht P (1994) Properties of the VIP-PACAP type II receptor stably expressed in CHO cells. Regul Pept 54:397–407
Ciccarelli E, Svoboda M, De NP, Di PE, Bollen A, Dubeaux C, Vilardaga JP, Waelbroeck M, Robberecht P (1995) Pharmacological properties of two recombinant splice variants of the PACAP type I receptor, transfected and stably expressed in CHO cells. Eur J Pharmacol 288:259–267
Ciranna L, Cavallaro S (2003) Opposing effects by pituitary adenylate cyclase-activating polypeptide and vasoactive intestinal peptide on hippocampal synaptic transmission. Exp Neurol 184:778–784
Claing A, Perry SJ, Achiriloaie M, Walker JK, Albanesi JP, Lefkowitz RJ, Premont RT (2000) Multiple endocytic pathways of G protein-coupled receptors delineated by GIT1 sensitivity. Proc Natl Acad Sci USA 97:1119–1124
Costa L, Santangelo F, Li VG, Ciranna L (2009) Modulation of AMPA receptor-mediated ion current by pituitary adenylate cyclase-activating polypeptide (PACAP) in CA1 pyramidal neurons from rat hippocampus. Hippocampus 19:99–109
Cull-Candy SG, Leszkiewicz DN (2004) Role of distinct NMDA receptor subtypes at central synapses. Sci STKE 2004:16
Cunha-Reis D, Sebastiao AM, Wirkner K, Illes P, Ribeiro JA (2004) VIP enhances both pre- and postsynaptic GABAergic transmission to hippocampal interneurones leading to increased excitatory synaptic transmission to CA1 pyramidal cells. Br J Pharmacol 143:733–744
Cunha-Reis D, Ribeiro JA, Sebastiao AM (2005) VIP enhances synaptic transmission to hippocampal CA1 pyramidal cells through activation of both VPAC1 and VPAC2 receptors. Brain Res 1049:52–60
Cunha-Reis D, Fontinha BM, Ribeiro JA, Sebastiao AM (2007) Tonic adenosine A1 and A2A receptor activation is required for the excitatory action of VIP on synaptic transmission in the CA1 area of the hippocampus. Neuropharmacology 52:313–320
Dautzenberg FM, Hauger RL (2001) G-protein-coupled receptor kinase 3- and protein kinase C-mediated desensitization of the PACAP receptor type 1 in human Y-79 retinoblastoma cells. Neuropharmacology 40:394–407
Delcourt N, Thouvenot E, Chanrion B, Galeotti N, Jouin P, Bockaert J, Marin P (2007) PACAP type I receptor transactivation is essential for IGF-1 receptor signalling and antiapoptotic activity in neurons. EMBO J 26:1542–1551
Dickson L, Finlayson K (2009) VPAC and PAC receptors: from ligands to function. Pharmacol Ther 121:294–316
Dickson L, Aramori I, McCulloch J, Sharkey J, Finlayson K (2006) A systematic comparison of intracellular cyclic AMP and calcium signalling highlights complexities in human VPAC/PAC receptor pharmacology. Neuropharmacology 51:1086–1098
Feany MB, Quinn WG (1995) A neuropeptide gene defined by the Drosophila memory mutant amnesiac. Science 268:869–873
Gaudin P, Couvineau A, Maoret JJ, Rouyer-Fessard C, Laburthe M (1996) Stable expression of the recombinant human VIP1 receptor in clonal Chinese hamster ovary cells: pharmacological, functional and molecular properties. Eur J Pharmacol 302:207–214
Glowa JR, Panlilio LV, Brenneman DE, Gozes I, Fridkin M, Hill JM (1992) Learning impairment following intracerebral administration of the HIV envelope protein gp120 or a VIP antagonist. Brain Res 570:49–53
Gozes I, Glowa J, Brenneman DE, McCune SK, Lee E, Westphal H (1993) Learning and sexual deficiencies in transgenic mice carrying a chimeric vasoactive intestinal peptide gene. J Mol Neurosci 4:185–193
Gozes I, Bardea A, Reshef A, Zamostiano R, Zhukovsky S, Rubinraut S, Fridkin M, Brenneman DE (1996) Neuroprotective strategy for Alzheimer disease: intranasal administration of a fatty neuropeptide. Proc Natl Acad Sci USA 93:427–432
Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L, Choquet D (2006) NMDA receptor surface mobility depends on NR2A-2B subunits. Proc Natl Acad Sci USA 103:18769–18774
Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP, Wank SA, Waschek JA (1998) International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev 50:265–270
Joo KM, Chung YH, Kim MK, Nam RH, Lee BL, Lee KH, Cha CI (2004) Distribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PAC1 receptor) in the rat brain. J Comp Neurol 476:388–413
Kondo T, Tominaga T, Ichikawa M, Iijima T (1997) Differential alteration of hippocampal synaptic strength induced by pituitary adenylate cyclase activating polypeptide-38 (PACAP-38). Neurosci Lett 221:189–192
Kotecha SA, Macdonald JF (2003) Signaling molecules and receptor transduction cascades that regulate NMDA receptor-mediated synaptic transmission. Int Rev Neurobiol 54:51–106
Kotecha SA, Jackson MF, Al-Mahrouki A, Roder JC, Orser BA, Macdonald JF (2003) Co-stimulation of mGluR5 and N-methyl-D-aspartate receptors is required for potentiation of excitatory synaptic transmission in hippocampal neurons. J Biol Chem 278:27742–27749
Lau CG, Zukin RS (2007) NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci 8:413–426
Lau CG, Takeuchi K, Rodenas-Ruano A, Takayasu Y, Murphy J, Bennett MV, Zukin RS (2009) Regulation of NMDA receptor Ca2+ signalling and synaptic plasticity. Biochem Soc Trans 37:1369–1374
Lerner EA, Iuga AO, Reddy VB (2007) Maxadilan, a PAC1 receptor agonist from sand flies. Peptides 28:1651–1654
Liu GJ, Madsen BW (1997) PACAP38 modulates activity of NMDA receptors in cultured chick cortical neurons. J Neurophysiol 78:2231–2234
Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT (2004) Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 304:1021–1024
Lu WY, Jackson MF, Bai D, Orser BA, Macdonald JF (2000) In CA1 pyramidal neurons of the hippocampus protein kinase C regulates calcium-dependent inactivation of NMDA receptors. J Neurosci 20:4452–4461
Macdonald JF, Kotecha SA, Lu WY, Jackson MF (2001) Convergence of PKC-dependent kinase signal cascades on NMDA receptors. Curr Drug Targets 2:299–312
Macdonald DS, Weerapura M, Beazely MA, Martin L, Czerwinski W, Roder JC, Orser BA, Macdonald JF (2005) Modulation of NMDA receptors by pituitary adenylate cyclase activating peptide in CA1 neurons requires G alpha q, protein kinase C, and activation of Src. J Neurosci 25:11374–11384
Malenka RC, Bear MF (2004) LTP and LTD: an embarrassment of riches. Neuron 44:5–21
Malinow R, Malenka RC (2002) AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25:103–126
Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI (2004) Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci 24:7821–7828
McCulloch DA, Lutz EM, Johnson MS, MacKenzie CJ, Mitchell R (2000) Differential activation of phospholipase D by VPAC and PAC1 receptors. Ann NY Acad Sci 921:175–185
McDonald TP, Dinnis DM, Morrison CF, Harmar AJ (1998) Desensitization of the human vasoactive intestinal peptide receptor (hVIP2/PACAP R): evidence for agonist-induced receptor phosphorylation and internalization. Ann NY Acad Sci 865:64–72
Michel S, Itri J, Han JH, Gniotczynski K, Colwell CS (2006) Regulation of glutamatergic signalling by PACAP in the mammalian suprachiasmatic nucleus. BMC Neurosci 7:15
Morishita W, Lu W, Smith GB, Nicoll RA, Bear MF, Malenka RC (2007) Activation of NR2B-containing NMDA receptors is not required for NMDA receptor-dependent long-term depression. Neuropharmacology 52:71–76
Neyton J, Paoletti P (2006) Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci 26:1331–1333
Otto C, Kovalchuk Y, Wolfer DP, Gass P, Martin M, Zuschratter W, Grone HJ, Kellendonk C, Tronche F, Maldonado R, Lipp HP, Konnerth A, Schutz G (2001) Impairment of mossy fiber long-term potentiation and associative learning in pituitary adenylate cyclase activating polypeptide type I receptor-deficient mice. J Neurosci 21:5520–5527
Roberto M, Brunelli M (2000) PACAP-38 enhances excitatory synaptic transmission in the rat hippocampal CA1 region. Learn Mem 7:303–311
Roberto M, Scuri R, Brunelli M (2001) Differential effects of PACAP-38 on synaptic responses in rat hippocampal CA1 region. Learn Mem 8:265–271
Sacchetti B, Lorenzini CA, Baldi E, Bucherelli C, Roberto M, Tassoni G, Brunelli M (2001) Pituitary adenylate cyclase-activating polypeptide hormone (PACAP) at very low dosages improves memory in the rat. Neurobiol Learn Mem 76:1–6
Said SI (2007) The discovery of VIP: initially looked for in the lung, isolated from intestine, and identified as a neuropeptide. Peptides 28:1620–1621
Salter MW, Kalia LV (2004) Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci 5:317–328
Shintani N, Hashimoto H, Kunugi A, Koyama Y, Yamamoto K, Tomimoto S, Mori W, Matsuda T, Baba A (2000) Desensitization, surface expression, and glycosylation of a functional, epitope-tagged type I PACAP (PAC(1)) receptor. Biochim Biophys Acta 1509:195–202
Skeberdis VA, Chevaleyre V, Lau CG, Goldberg JH, Pettit DL, Suadicani SO, Lin Y, Bennett MV, Yuste R, Castillo PE, Zukin RS (2006) Protein kinase A regulates calcium permeability of NMDA receptors. Nat Neurosci 9:501–510
Ster J, de Bock F, Bertaso F, Abitbol K, Daniel H, Bockaert J, Fagni L (2009) Epac mediates PACAP-dependent long-term depression in the hippocampus. J Physiol 587:101–113
Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P (2004) DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol 44:269–296
Weitlauf C, Honse Y, Auberson YP, Mishina M, Lovinger DM, Winder DG (2005) Activation of NR2A-containing NMDA receptors is not obligatory for NMDA receptor-dependent long-term potentiation. J Neurosci 25:8386–8390
Wu SY, Dun NJ (1997) Potentiation of NMDA currents by pituitary adenylate cyclase activating polypeptide in neonatal rat sympathetic preganglionic neurons. J Neurophysiol 78:1175–1179
Yaka R, He DY, Phamluong K, Ron D (2003) Pituitary adenylate cyclase-activating polypeptide (PACAP(1–38)) enhances N-methyl-D-aspartate receptor function and brain-derived neurotrophic factor expression via RACK1. J Biol Chem 278:9630–9638
Yang K, Trepanier CH, Li H, Beazely MA, Lerner EA, Jackson MF, Macdonald JF (2009) Vasoactive intestinal peptide acts via multiple signal pathways to regulate hippocampal NMDA receptors and synaptic transmission. Hippocampus 19:779–789
Yashiro K, Philpot BD (2008) Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology 55:1081–1094
Disclosure statement
The authors have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants to JFM from the Canadian Institutes of Health Research (15514 and 44008).
Rights and permissions
About this article
Cite this article
Yang, K., Lei, G., Jackson, M.F. et al. The Involvement of PACAP/VIP System in the Synaptic Transmission in the Hippocampus. J Mol Neurosci 42, 319–326 (2010). https://doi.org/10.1007/s12031-010-9372-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-010-9372-7