Abstract
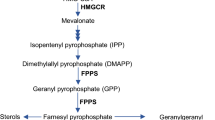
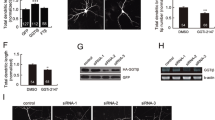
Protein prenylation is a post-translational lipid modification that governs a variety of important cellular signaling pathways, including those regulating synaptic functions and cognition in the nervous system. Two enzymes, farnesyltransferase (FT) and geranylgeranyltransferase type I (GGT), are essential for the prenylation process. Genetic reduction of FT or GGT ameliorates neuropathology but only FT haplodeficiency rescues cognitive function in transgenic mice of Alzheimer’s disease. A follow-up study showed that systemic or forebrain neuron-specific deficiency of GGT leads to synaptic and cognitive deficits under physiological conditions. Whether FT plays different roles in shaping neuronal functions and cognition remains elusive. This study shows that in contrast to the detrimental effects of GGT reduction, systemic haplodeficiency of FT has little to no impact on hippocampal synaptic plasticity and cognition. However, forebrain neuron-specific FT deletion also leads to reduced synaptic plasticity, memory retention, and hippocampal dendritic spine density. Furthermore, a novel prenylomic analysis identifies distinct pools of prenylated proteins that are affected in the brain of forebrain neuron-specific FT and GGT knockout mice, respectively. Taken together, this study uncovers that physiological levels of FT and GGT in neurons are essential for normal synaptic/cognitive functions and that the prenylation status of specific signaling molecules regulates neuronal functions.








Similar content being viewed by others
Data availability
Raw data files are available upon request.
References
Lane KT, Beese LS (2006) Thematic review series: lipid posttranslational modifications. Structural biology of protein farnesyltransferase and geranylgeranyltransferase type I. J Lipid Res 47(4):681–699. https://doi.org/10.1194/jlr.R600002-JLR200
Li H, Kuwajima T, Oakley D, Nikulina E, Hou J, Yang WS, Lowry ER, Lamas NJ et al (2016) Protein prenylation constitutes an endogenous brake on axonal growth. Cell Rep 16(2):545–558. https://doi.org/10.1016/j.celrep.2016.06.013
Jeong A, Suazo KF, Wood WG, Distefano MD, Li L (2018) Isoprenoids and protein prenylation: implications in the pathogenesis and therapeutic intervention of Alzheimer’s disease. Crit Rev Biochem Mol Biol 53(3):279–310. https://doi.org/10.1080/10409238.2018.1458070
Lee HJ, Kang SJ, Lee K, Im H (2011) Human α-synuclein modulates vesicle trafficking through its interaction with prenylated Rab acceptor protein 1. Biochem Biophys Res Commun 412(4):526–531
Goldstein JL, Brown MS (1990) Regulation of the mevalonate pathway. Nature 343(6257):425–430. https://doi.org/10.1038/343425a0
McTaggart SJ (2006) Isoprenylated proteins. Cell Mol Life Sci 63(3):255–267. https://doi.org/10.1007/s00018-005-5298-6
Gao J, Liao J, Yang GY (2009) CAAX-box protein, prenylation process and carcinogenesis. Am J Transl Res 1(3):312–325
Kinsella BT, Maltese WA (1992) rab GTP-binding proteins with three different carboxyl-terminal cysteine motifs are modified in vivo by 20-carbon isoprenoids. J Biol Chem 267(6):3940–3945
Kuchay S, Wang H, Marzio A, Jain K, Homer H, Fehrenbacher N, Philips MR, Zheng N et al (2019) GGTase3 is a newly identified geranylgeranyltransferase targeting a ubiquitin ligase. Nat Struct Mol Biol 26(7):628–636
Shirakawa R, Goto-Ito S, Goto K, Wakayama S, Kubo H, Sakata N, Trinh DA, Yamagata A et al (2020) A SNARE geranylgeranyltransferase essential for the organization of the Golgi apparatus. EMBO J 39(8):e104120. https://doi.org/10.15252/embj.2019104120
Hooff GP, Wood WG, Muller WE, Eckert GP (2010) Isoprenoids, small GTPases and Alzheimer’s disease. Biochim Biophys Acta 1801(8):896–905. https://doi.org/10.1016/j.bbalip.2010.03.014
Hottman DA, Li L (2014) Protein prenylation and synaptic plasticity: implications for Alzheimer’s disease. Mol Neurobiol 50(1):177–185. https://doi.org/10.1007/s12035-013-8627-z
Ryu HH, Lee YS (2016) Cell type-specific roles of RAS-MAPK signaling in learning and memory: implications in neurodevelopmental disorders. Neurobiol Learn Mem 135:13–21. https://doi.org/10.1016/j.nlm.2016.06.006
Ye X, Carew TJ (2010) Small G protein signaling in neuronal plasticity and memory formation: the specific role of ras family proteins. Neuron 68(3):340–361. https://doi.org/10.1016/j.neuron.2010.09.013
Takai Y, Sasaki T, Matozaki T (2001) Small GTP-binding proteins. Physiol Rev 81(1):153–208. https://doi.org/10.1152/physrev.2001.81.1.153
Li L, Zhang W, Cheng S, Cao D, Parent M (2012) Isoprenoids and related pharmacological interventions: potential application in Alzheimer’s disease. Mol Neurobiol 46(1):64–77. https://doi.org/10.1007/s12035-012-8253-1
Cheng S, Cao D, Hottman DA, Yuan L, Bergo MO, Li L (2013) Farnesyltransferase haplodeficiency reduces neuropathology and rescues cognitive function in a mouse model of Alzheimer disease. J Biol Chem 288(50):35952–35960. https://doi.org/10.1074/jbc.M113.503904
Hottman D, Cheng S, Gram A, LeBlanc K, Yuan LL, Li L (2018) Systemic or forebrain neuron-specific deficiency of geranylgeranyltransferase-1 impairs synaptic plasticity and reduces dendritic spine density. Neuroscience 373:207–217. https://doi.org/10.1016/j.neuroscience.2018.01.026
Liu M, Sjogren AK, Karlsson C, Ibrahim MX, Andersson KM, Olofsson FJ, Wahlstrom AM, Dalin M et al (2010) Targeting the protein prenyltransferases efficiently reduces tumor development in mice with K-RAS-induced lung cancer. Proc Natl Acad Sci U S A 107(14):6471–6476. https://doi.org/10.1073/pnas.0908396107
Sjogren AK, Andersson KM, Liu M, Cutts BA, Karlsson C, Wahlstrom AM, Dalin M, Weinbaum C et al (2007) GGTase-I deficiency reduces tumor formation and improves survival in mice with K-RAS-induced lung cancer. J Clin Invest 117(5):1294–1304. https://doi.org/10.1172/JCI30868
Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER et al (1996) Subregion- and cell type-restricted gene knockout in mouse brain. Cell 87(7):1317–1326. https://doi.org/10.1016/s0092-8674(00)81826-7
Parent MA, Hottman DA, Cheng S, Zhang W, McMahon LL, Yuan LL, Li L (2014) Simvastatin treatment enhances NMDAR-mediated synaptic transmission by upregulating the surface distribution of the GluN2B subunit. Cell Mol Neurobiol 34(5):693–705. https://doi.org/10.1007/s10571-014-0051-z
Dumanis SB, Tesoriero JA, Babus LW, Nguyen MT, Trotter JH, Ladu MJ, Weeber EJ, Turner RS et al (2009) ApoE4 decreases spine density and dendritic complexity in cortical neurons in vivo. J Neurosci 29(48):15317–15322. https://doi.org/10.1523/JNEUROSCI.4026-09.2009
Adlard PA, Bica L, White AR, Nurjono M, Filiz G, Crouch PJ, Donnelly PS, Cappai R et al (2011) Metal ionophore treatment restores dendritic spine density and synaptic protein levels in a mouse model of Alzheimer’s disease. PLoS One 6(3):e17669. https://doi.org/10.1371/journal.pone.0017669
Brandt AC, McNally L, Lorimer EL, Unger B, Koehn OJ, Suazo KF, Rein L, Szabo A et al (2020) Splice switching an oncogenic ratio of SmgGDS isoforms as a strategy to diminish malignancy. Proc Natl Acad Sci U S A 117(7):3627–3636. https://doi.org/10.1073/pnas.1914153117
Suazo KF, Schaber C, Palsuledesai CC, John ARO, Distefano MD (2016) Global proteomic analysis of prenylated proteins in Plasmodium falciparum using an alkyne-modified isoprenoid analogue. Sci Rep 6:38615
McAlister GC, Nusinow DP, Jedrychowski MP, Wuhr M, Huttlin EL, Erickson BK, Rad R, Haas W et al (2014) MultiNotch MS3 enables accurate, sensitive, and multiplexed detection of differential expression across cancer cell line proteomes. Anal Chem 86(14):7150–7158. https://doi.org/10.1021/ac502040v
Kumar A (2011) Long-term potentiation at CA3–CA1 hippocampal synapses with special emphasis on aging, disease, and stress. Front Aging Neurosci 3:7
Okada T, Yamada N, Tsuzuki K, Horikawa HP, Tanaka K, Ozawa S (2003) Long-term potentiation in the hippocampal CA1 area and dentate gyrus plays different roles in spatial learning. Eur J Neurosci 17(2):341–349. https://doi.org/10.1046/j.1460-9568.2003.02458.x
Tsien JZ, Huerta PT, Tonegawa S (1996) The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 87(7):1327–1338. https://doi.org/10.1016/s0092-8674(00)81827-9
McGaugh JL (2000) Memory--a century of consolidation. Science 287(5451):248–251. https://doi.org/10.1126/science.287.5451.248
Regehr WG (2012) Short-term presynaptic plasticity. Cold Spring Harb Perspect Biol 4(7):a005702. https://doi.org/10.1101/cshperspect.a005702
Mijimolle N, Velasco J, Dubus P, Guerra C, Weinbaum CA, Casey PJ, Campuzano V, Barbacid M (2005) Protein farnesyltransferase in embryogenesis, adult homeostasis, and tumor development. Cancer Cell 7(4):313–324. https://doi.org/10.1016/j.ccr.2005.03.004
Liu Q, Trotter J, Zhang J, Peters MM, Cheng H, Bao J, Han X, Weeber EJ et al (2010) Neuronal LRP1 knockout in adult mice leads to impaired brain lipid metabolism and progressive, age-dependent synapse loss and neurodegeneration. J Neurosci 30(50):17068–17078. https://doi.org/10.1523/JNEUROSCI.4067-10.2010
DeGraw AJ, Palsuledesai C, Ochocki JD, Dozier JK, Lenevich S, Rashidian M, Distefano MD (2010) Evaluation of alkyne-modified isoprenoids as chemical reporters of protein prenylation. Chem Biol Drug Des 76(6):460–471
Zhang Y, Blanden MJ, Sudheer C, Gangopadhyay SA, Rashidian M, Hougland JL, Distefano MD (2015) Simultaneous site-specific dual protein labeling using protein prenyltransferases. Bioconjug Chem 26(12):2542–2553
Mu R, Dussupt V, Jiang J, Sette P, Rudd V, Chuenchor W, Bello NF, Bouamr F et al (2012) Two distinct binding modes define the interaction of Brox with the C-terminal tails of CHMP5 and CHMP4B. Structure 20(5):887–898. https://doi.org/10.1016/j.str.2012.03.001
Marschang P, Brich J, Weeber EJ, Sweatt JD, Shelton JM, Richardson JA, Hammer RE, Herz J (2004) Normal development and fertility of knockout mice lacking the tumor suppressor gene LRP1b suggest functional compensation by LRP1. Mol Cell Biol 24(9):3782–3793. https://doi.org/10.1128/mcb.24.9.3782-3793.2004
Orthwein A, Zahn A, Methot SP, Godin D, Conticello SG, Terada K, Di Noia JM (2012) Optimal functional levels of activation-induced deaminase specifically require the Hsp40 DnaJa1. EMBO J 31(3):679–691. https://doi.org/10.1038/emboj.2011.417
Rauch JN, Gestwicki JE (2014) Binding of human nucleotide exchange factors to heat shock protein 70 (Hsp70) generates functionally distinct complexes in vitro. J Biol Chem 289(3):1402–1414. https://doi.org/10.1074/jbc.M113.521997
Imai Y, Soda M, Hatakeyama S, Akagi T, Hashikawa T, Nakayama KI, Takahashi R (2002) CHIP is associated with Parkin, a gene responsible for familial Parkinson’s disease, and enhances its ubiquitin ligase activity. Mol Cell 10(1):55–67. https://doi.org/10.1016/s1097-2765(02)00583-x
Gotoh T, Terada K, Oyadomari S, Mori M (2004) hsp70-DnaJ chaperone pair prevents nitric oxide- and CHOP-induced apoptosis by inhibiting translocation of Bax to mitochondria. Cell Death Differ 11(4):390–402. https://doi.org/10.1038/sj.cdd.4401369
Hageman J, van Waarde MA, Zylicz A, Walerych D, Kampinga HH (2011) The diverse members of the mammalian HSP70 machine show distinct chaperone-like activities. Biochem J 435(1):127–142. https://doi.org/10.1042/BJ20101247
Edwards MC, Liegeois N, Horecka J, DePinho RA, Sprague GF Jr, Tyers M, Elledge SJ (1997) Human CPR (cell cycle progression restoration) genes impart a Far- phenotype on yeast cells. Genetics 147(3):1063–1076
Pencheva N, Tran H, Buss C, Huh D, Drobnjak M, Busam K, Tavazoie SF (2012) Convergent multi-miRNA targeting of ApoE drives LRP1/LRP8-dependent melanoma metastasis and angiogenesis. Cell 151(5):1068–1082. https://doi.org/10.1016/j.cell.2012.10.028
Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD et al (2005) Towards a proteome-scale map of the human protein-protein interaction network. Nature 437(7062):1173–1178. https://doi.org/10.1038/nature04209
Rolland T, Tasan M, Charloteaux B, Pevzner SJ, Zhong Q, Sahni N, Yi S, Lemmens I et al (2014) A proteome-scale map of the human interactome network. Cell 159(5):1212–1226. https://doi.org/10.1016/j.cell.2014.10.050
Huttlin EL, Bruckner RJ, Paulo JA, Cannon JR, Ting L, Baltier K, Colby G, Gebreab F et al (2017) Architecture of the human interactome defines protein communities and disease networks. Nature 545(7655):505–509. https://doi.org/10.1038/nature22366
Gaudet P, Livstone MS, Lewis SE, Thomas PD (2011) Phylogenetic-based propagation of functional annotations within the Gene Ontology consortium. Brief Bioinform 12(5):449–462. https://doi.org/10.1093/bib/bbr042
O’Leary NA, Wright MW, Brister JR, Ciufo S, Haddad D, McVeigh R, Rajput B, Robbertse B et al (2016) Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res 44(D1):D733–D745. https://doi.org/10.1093/nar/gkv1189
Simon HU, Mills GB, Kozlowski M, Hogg D, Branch D, Ishimi Y, Siminovitch KA (1994) Molecular characterization of hNRP, a cDNA encoding a human nucleosome-assembly-protein-I-related gene product involved in the induction of cell proliferation. Biochem J 297(Pt 2):389–397. https://doi.org/10.1042/bj2970389
Rodriguez P, Munroe D, Prawitt D, Chu LL, Bric E, Kim J, Reid LH, Davies C et al (1997) Functional characterization of human nucleosome assembly protein-2 (NAP1L4) suggests a role as a histone chaperone. Genomics 44(3):253–265. https://doi.org/10.1006/geno.1997.4868
Jones JM, Morrell JC, Gould SJ (2004) PEX19 is a predominantly cytosolic chaperone and import receptor for class 1 peroxisomal membrane proteins. J Cell Biol 164(1):57–67. https://doi.org/10.1083/jcb.200304111
Jones JM, Morrell JC, Gould SJ (2001) Multiple distinct targeting signals in integral peroxisomal membrane proteins. J Cell Biol 153(6):1141–1150. https://doi.org/10.1083/jcb.153.6.1141
Delille HK, Schrader M (2008) Targeting of hFis1 to peroxisomes is mediated by Pex19p. J Biol Chem 283(45):31107–31115. https://doi.org/10.1074/jbc.M803332200
Sugiura H, Yasuda S, Katsurabayashi S, Kawano H, Endo K, Takasaki K, Iwasaki K, Ichikawa M et al (2015) Rheb activation disrupts spine synapse formation through accumulation of syntenin in tuberous sclerosis complex. Nat Commun 6:6842. https://doi.org/10.1038/ncomms7842
Yamagata K, Sanders LK, Kaufmann WE, Yee W, Barnes CA, Nathans D, Worley PF (1994) rheb, a growth factor- and synaptic activity-regulated gene, encodes a novel Ras-related protein. J Biol Chem 269(23):16333–16339
Brown HL, Kaun KR, Edgar BA (2012) The small GTPase Rheb affects central brain neuronal morphology and memory formation in Drosophila. PLoS One 7(9):e44888. https://doi.org/10.1371/journal.pone.0044888
Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J (2003) Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol 13(15):1259–1268. https://doi.org/10.1016/s0960-9822(03)00506-2
Swanger SA, Mattheyses AL, Gentry EG, Herskowitz JH (2015) ROCK1 and ROCK2 inhibition alters dendritic spine morphology in hippocampal neurons. Cell Logist 5(4):e1133266. https://doi.org/10.1080/21592799.2015.1133266
Henderson BW, Greathouse KM, Ramdas R, Walker CK, Rao TC, Bach SV, Curtis KA, Day JJ et al (2019) Pharmacologic inhibition of LIMK1 provides dendritic spine resilience against beta-amyloid. Sci Signal 12(587):eaaw9318. https://doi.org/10.1126/scisignal.aaw9318
Greathouse KM, Boros BD, Deslauriers JF, Henderson BW, Curtis KA, Gentry EG, Herskowitz JH (2018) Distinct and complementary functions of rho kinase isoforms ROCK1 and ROCK2 in prefrontal cortex structural plasticity. Brain Struct Funct 223(9):4227–4241. https://doi.org/10.1007/s00429-018-1748-4
Kozma R, Sarner S, Ahmed S, Lim L (1997) Rho family GTPases and neuronal growth cone remodelling: relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol Cell Biol 17(3):1201–1211. https://doi.org/10.1128/mcb.17.3.1201
Suzukawa K, Miura K, Mitsushita J, Resau J, Hirose K, Crystal R, Kamata T (2000) Nerve growth factor-induced neuronal differentiation requires generation of Rac1-regulated reactive oxygen species. J Biol Chem 275(18):13175–13178. https://doi.org/10.1074/jbc.275.18.13175
Tashiro A, Yuste R (2004) Regulation of dendritic spine motility and stability by Rac1 and Rho kinase: evidence for two forms of spine motility. Mol Cell Neurosci 26(3):429–440. https://doi.org/10.1016/j.mcn.2004.04.001
Wiens KM, Lin H, Liao D (2005) Rac1 induces the clustering of AMPA receptors during spinogenesis. J Neurosci 25(46):10627–10636. https://doi.org/10.1523/JNEUROSCI.1947-05.2005
Schwindinger WF, Giger KE, Betz KS, Stauffer AM, Sunderlin EM, Sim-Selley LJ, Selley DE, Bronson SK et al (2004) Mice with deficiency of G protein gamma3 are lean and have seizures. Mol Cell Biol 24(17):7758–7768. https://doi.org/10.1128/MCB.24.17.7758-7768.2004
Dasgupta S, Cushman I, Kpetemey M, Casey PJ, Vishwanatha JK (2011) Prenylated c17orf37 induces filopodia formation to promote cell migration and metastasis. J Biol Chem 286(29):25935–25946. https://doi.org/10.1074/jbc.M111.254599
Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K et al (1999) Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 285(5429):895–898. https://doi.org/10.1126/science.285.5429.895
Troca-Marin JA, Alves-Sampaio A, Tejedor FJ, Montesinos ML (2010) Local translation of dendritic RhoA revealed by an improved synaptoneurosome preparation. Mol Cell Neurosci 43(3):308–314. https://doi.org/10.1016/j.mcn.2009.12.004
Chen Y, Yang Z, Meng M, Zhao Y, Dong N, Yan H, Liu L, Ding M et al (2009) Cullin mediates degradation of RhoA through evolutionarily conserved BTB adaptors to control actin cytoskeleton structure and cell movement. Mol Cell 35(6):841–855. https://doi.org/10.1016/j.molcel.2009.09.004
Chen Y, Kramar EA, Chen LY, Babayan AH, Andres AL, Gall CM, Lynch G, Baram TZ (2013) Impairment of synaptic plasticity by the stress mediator CRH involves selective destruction of thin dendritic spines via RhoA signaling. Mol Psychiatry 18(4):485–496. https://doi.org/10.1038/mp.2012.17
Briz V, Zhu G, Wang Y, Liu Y, Avetisyan M, Bi X, Baudry M (2015) Activity-dependent rapid local RhoA synthesis is required for hippocampal synaptic plasticity. J Neurosci 35(5):2269–2282. https://doi.org/10.1523/JNEUROSCI.2302-14.2015
Wang X, Zhang C, Szabo G, Sun QQ (2013) Distribution of CaMKIIalpha expression in the brain in vivo, studied by CaMKIIalpha-GFP mice. Brain Res 1518:9–25. https://doi.org/10.1016/j.brainres.2013.04.042
Choi CI, Yoon SP, Choi JM, Kim SS, Lee YD, Birnbaumer L, Suh-Kim H (2014) Simultaneous deletion of floxed genes mediated by CaMKIIalpha-Cre in the brain and in male germ cells: application to conditional and conventional disruption of Goalpha. Exp Mol Med 46(5):e93. https://doi.org/10.1038/emm.2014.14
Minichiello L, Korte M, Wolfer D, Kuhn R, Unsicker K, Cestari V, Rossi-Arnaud C, Lipp HP et al (1999) Essential role for TrkB receptors in hippocampus-mediated learning. Neuron 24(2):401–414. https://doi.org/10.1016/s0896-6273(00)80853-3
Hernandez I, Luna G, Rauch JN, Reis SA, Giroux M, Karch CM, Boctor D, Sibih YE et al (2019) A farnesyltransferase inhibitor activates lysosomes and reduces tau pathology in mice with tauopathy. Sci Transl Med 11(485):eaat3005. https://doi.org/10.1126/scitranslmed.aat3005
Gao S, Yu R, Zhou X (2016) The role of geranylgeranyltransferase I-mediated protein prenylation in the brain. Mol Neurobiol 53(10):6925–6937. https://doi.org/10.1007/s12035-015-9594-3
Schultz BG, Patten DK, Berlau DJ (2018) The role of statins in both cognitive impairment and protection against dementia: a tale of two mechanisms. Transl Neurodegener 7:5. https://doi.org/10.1186/s40035-018-0110-3
Mans RA, McMahon LL, Li L (2012) Simvastatin-mediated enhancement of long-term potentiation is driven by farnesyl-pyrophosphate depletion and inhibition of farnesylation. Neuroscience 202:1–9
Storck EM, Morales-Sanfrutos J, Serwa RA, Panyain N, Lanyon-Hogg T, Tolmachova T, Ventimiglia LN, Martin-Serrano J et al (2019) Dual chemical probes enable quantitative system-wide analysis of protein prenylation and prenylation dynamics. Nat Chem 11(6):552–561. https://doi.org/10.1038/s41557-019-0237-6
Roskoski R Jr, Ritchie P (1998) Role of the carboxyterminal residue in peptide binding to protein farnesyltransferase and protein geranylgeranyltransferase. Arch Biochem Biophys 356(2):167–176
Winter-Vann AM, Casey PJ (2005) Post-prenylation-processing enzymes as new targets in oncogenesis. Nat Rev Cancer 5(5):405–412
Nguyen UT, Guo Z, Delon C, Wu Y, Deraeve C, Franzel B, Bon RS, Blankenfeldt W et al (2009) Analysis of the eukaryotic prenylome by isoprenoid affinity tagging. Nat Chem Biol 5(4):227–235. https://doi.org/10.1038/nchembio.149
Ali N, Jurczyluk J, Shay G, Tnimov Z, Alexandrov K, Munoz MA, Skinner OP, Pavlos NJ et al (2015) A highly sensitive prenylation assay reveals in vivo effects of bisphosphonate drug on the Rab prenylome of macrophages outside the skeleton. Small GTPases 6(4):202–211. https://doi.org/10.1080/21541248.2015.1085485
Kohnke M, Delon C, Hastie ML, Nguyen UT, Wu YW, Waldmann H, Goody RS, Gorman JJ et al (2013) Rab GTPase prenylation hierarchy and its potential role in choroideremia disease. PLoS One 8(12):e81758. https://doi.org/10.1371/journal.pone.0081758
Hanker AB, Mitin N, Wilder RS, Henske EP, Tamanoi F, Cox AD, Der CJ (2010) Differential requirement of CAAX-mediated posttranslational processing for Rheb localization and signaling. Oncogene 29(3):380–391. https://doi.org/10.1038/onc.2009.336
Buerger C, DeVries B, Stambolic V (2006) Localization of Rheb to the endomembrane is critical for its signaling function. Biochem Biophys Res Commun 344(3):869–880. https://doi.org/10.1016/j.bbrc.2006.03.220
Schwartz M (2004) Rho signalling at a glance. J Cell Sci 117(23):5457–5458
Liu Z, Meray RK, Grammatopoulos TN, Fredenburg RA, Cookson MR, Liu Y, Logan T, Lansbury PT (2009) Membrane-associated farnesylated UCH-L1 promotes α-synuclein neurotoxicity and is a therapeutic target for Parkinson’s disease. Proc Natl Acad Sci 106(12):4635–4640
Suazo KF, Hurben AK, Liu K, Xu F, Thao P, Sudheer C, Li L, Distefano MD (2018) Metabolic labeling of prenylated proteins using alkyne-modified isoprenoid analogues. Curr Protoc Chem Biol 10(3):e46
Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M (2011) Global quantification of mammalian gene expression control. Nature 473(7347):337–342. https://doi.org/10.1038/nature10098
Schuld NJ, Vervacke JS, Lorimer EL, Simon NC, Hauser AD, Barbieri JT, Distefano MD, Williams CL (2014) The chaperone protein SmgGDS interacts with small GTPases entering the prenylation pathway by recognizing the last amino acid in the CAAX motif. J Biol Chem 289(10):6862–6876. https://doi.org/10.1074/jbc.M113.527192
Acknowledgments
We thank Dr. Martin Bergo for providing the original breeding pairs for FT- and GGT-haplodeficient and floxed mice and Andrea Gram for maintaining and genotyping different lines of experimental mice.
Funding
This work was supported in part by grants from the National Institute on Aging of the National Institutes of Health (AG056976 and AG058081), National Institute of General Medical Science (R01GM084152), the National Institute of Neurological Disorders and Stroke (R01NS107442), and the National Science Foundation (CHE-1308655). KFS was supported by a Doctoral Dissertation Fellowship from the University of Minnesota.
Author information
Authors and Affiliations
Contributions
WQ performed electrophysiological experiments, behavioral assays for the FT+/- mice and their controls, analyzed the data, interpreted the results, and wrote the manuscript. KS performed prenylomic analysis and wrote the manuscript. WL conducted Golgi staining and quantification of dendritic spine density. SC performed behavioral assays for the FTf/fCre+ and FTf/fCre− mice. DH assisted with electrophysiological and Golgi staining experiments. AJ processed samples for the prenylomic analysis and provided suggestions on the manuscript. MD designed and supervised the prenylomic part of the study. LY contributed to the discussion and interpretation of the data from electrophysiological experiments. LL conceived the study, supervised the progress of all experiments, interpreted the results, and edited and finalized the manuscript
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Minnesota.
Consent to Participate
N/A
Consent for Publication
All authors have reviewed and approved the manuscript.
Code Availability
N/A
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 259 kb).
Rights and permissions
About this article
Cite this article
Qu, W., Suazo, K.F., Liu, W. et al. Neuronal Protein Farnesylation Regulates Hippocampal Synaptic Plasticity and Cognitive Function. Mol Neurobiol 58, 1128–1144 (2021). https://doi.org/10.1007/s12035-020-02169-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02169-w