Abstract
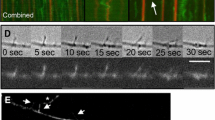
Axoplasmic organelles move on actin as well as microtubules in vitro and axons contain a large amount of actin, but little is known about the organization and distribution of actin filaments within the axon. Here we undertake to define the relationship of the microtubule bundles typically found in axons to actin filaments by applying three microscopic techniques: laser-scanning confocal microscopy of immuno-labeled squid axoplasm; electronmicroscopy of conventionally prepared thin sections; and electronmicroscopy of touch preparations-a thin layer of axoplasm transferred to a specimen grid and negatively stained. Light microscopy shows that longitudinal actin filaments are abundant and usually coincide with longitudinal microtubule bundles. Electron microscopy shows that microfilaments are interwoven with the longitudinal bundles of microtubules. These bundles maintain their integrity when neurofilaments are extracted. Some, though not all microfilaments decorate with the S1 fragment of myosin, and some also act as nucleation sites for polymerization of exogenous actin, and hence are definitively identified as actin filaments. These actin filaments range in minimum length from 0.5 to 1.5 µm with some at least as long as 3.5 µm. We conclude that the microtubule-based tracks for fast organelle transport also include actin filaments. These actin filaments are sufficiently long and abundant to be ancillary or supportive of fast transport along microtubules within bundles, or to extend transport outside of the bundle. These actin filaments could also be essential for maintaining the structural integrity of the microtubule bundles.
Similar content being viewed by others
References
BEARER E. L. (1990) Platelet membrane skeleton revealed by quick-freeze deep-etch. Anatomical Record 227, 1–11.
BEARER, E. L. (1992) An actin-associated protein present in the microtubule organizing center and the growth cone of PC-12 cells. Journal of Neuroscience 12, 750–61.
BEARER, E. L., DEGIORGIS, J. A., BODNER, R. A., KAO, A. W. & REESE, T. S. (1993) Evidence for myosin motors on organelles in squid axoplasm. Proceedings of the National Academy of Sciences USA 90, 11252–256.
BEARER, E. L., DEGIORGIS, J. A., JAFFE, H., MEDEIROS, N. A. & REESE, T. S. (1996) An axoplasmic myosin with a calmodulin-like light chain. Proceedings of the National Academy of Sciences USA 93, 6064–68.
BEARER, E. L., DEGIORGIS J. A. MEDEIROS N. A. & REESE T. S. (1996b) Actin-based motility of isolated axoplasmic organelles. Cell Motility and the Cytoskeleton 33, 106–14.
BEARER, E. L., LIU, J., HSU, A. & REESE, T. S. (1996c) Method for visualizing filaments in axoplasm by electron microscopy. Biological Bulletin 191, 272–73.
BRADY, S. T., LASEK, R. J., ALLEN, R. D., YIN, H.L. & STOSSEL, T. P. (1984) Gelsolin inhibition of fast axonal transport indicates a requirement for actin microfilaments. Nature 310, 56–8.
CUNNINGHAM, C. C., LECLERC, N., FLANAGAN, L. A., LU, M., JANMEY, P. A. & OSIK, K. S. (1997) otubule-associated protein 2c reorganizes both microtubules and microfilaments into distinct cytological structures in an actin-binding-protein-280-deficient melanoma cell line. Journal of Cell Biology 136, 57
DABORA S. L. & HEETZ M. P. (1988) The microtubuledependent formation of a tubulovesicular network with characteristics of the ER from cultured cell extracts. Cell 154, 27–35.
FATH, K. R., TRIMBUR, G. M. & BURGESS, D. R. (1994) Molecular motors are differentially distributed on Golgi membranes from polarized epithelial cells. Journal of Cell Biology 126, 661–75.
FATH, K. R. & LASEK, R. J. (1988) Two classes of actin microfilaments are associated with the inner cytoskeleton of axons. Journal of Cell Biology 107, 613–21.
GALLANT, P. E., PANT, H. C., PRUS S, R. M. & GAINER, H. (1986) Calcium-activated proteolysis of neurofilament proteins in the squid giant neuron. Journal of Neurochemistry 46, 1573–81.
GAVIN, R. H. (1997) Microtubule-microfilament synergy in the cytoskeleton. International Review of Cytology 173, 207–42.
KUCZMARSKI, E. R. & ROSENBAUM, J. L. (1979) Studies on the organization and localization of actin and myosin in neurons. Journal of Cell Biology 80, 356–71.
KUZNETSOV, S. A., LANGFORD, G. M. & WEISS, D. G. (1992) Actin-dependent organelle movement in squid axoplasm. Nature 356, 722–25.
LANGFORD, G. M. KUZNETSOV, S. A., JOHNSON, D., COHEN, D. L. & WEISS D. G. (1994) Movement of axoplasmic organelles on actin filaments assembled on acrosomal processes: Evidence for a barbed-end-directed organelle motor. Journal of Cell Science 107, 2291–98.
LEAPMAN, R. D., GALLANT, P. E., REESE, T. S. & ANDREWS, S. B. (1997) Phosphorylation and subunit organization of axonal neurofilaments determined by scanning transmission electron microscopy. Proceedings of the National Academy of Sciences USA 94, 7820–24.
MENZEL, D. & SCHLIWA, M. (1986a) Motility in the siphonous green alga Bryopsis. I. Spatial organization of the cytoskeleton and organelle movements. European Journal of Cell Biology 40, 275–85.
MENZEL, D. & SCHLIWA, M. (1986b) Motility in the siphonous green alga Bryopsis. II. Chloroplast movement requires organized arrays of both microtubules and actin filaments. European Journal of Cell Biology 40, 286–95.
METUZALS, J., CHANG, D., HAMMAR, K. & REESE, T. S. (1997) Organization of the cortical endoplasmic reticulum in the squid giant axon. Journal of Neurocytology 26, 529–39.
MOLYNEAUX, B. J. & LANGFORD, G. M. (1997) Characterization of antibodies to the head and tail domains of squid brain myosin V. Biology Bulletin 193, 222–23.
MORRIS R. L. & HOLLENBECK, P. J. (1995) Axonal transport of mitochondria along microtubules and F-actin in living vertebrate neurons. Journal of Cell Biology 131, 315–26
OLINK-COUX, M. & HOLLENBECK, P. J. (1996) Localization and active transport of mRNA in axons of sympathetic neurons in culture. Journal of Neurosciences 16, 1346–58.
MARTIN, R. (1996) The structure of the neurofilament cytoskeleton in the squid giant axon and synapse. Journal of Neurocytology 25, 547–54.
RODIONOV, V. I., HOPE, A. J., SVITKINA, T. M. & BORISY, G. G. (1998) Functional coordination of microtubule-based and actin-based motility in melanophores. Current Biology 8, 165–68.
ROGERS, S. L. & GELFAND, V. I. (1998) Myosin cooperates with microtubule motors during organelle transport in melanophores. Current Biology 8, 161–64.
SCHNAPP B. J., VALE, R. D., SHEETZ, M. P. & REESE, T. S. (1985) Single microtubules from squid axoplasm support bidirectional movement of organelles. Cell 40, 455–62.
SVITKINA, T. M., VERKHOVSKY, A. B. & BORISY, G. G. (1996) Plectin sidearms mediate interaction of intermediate filaments with microtubules and other components of the cytoskeleton. Journal of Cell Biology 135, 991–1007.
TABB, J. S., HARMON, K. O., DEPINA, A. S. & LANGFORD, G. M. (1996) Localization of myosin on tubulovesicular organelles in the squid giant axon by immuno-EM. Biology Bulletin 191, 274–75.
TILNEY, L. G., BONDER, E. M., & DEROSIER, D. J. (1981) Actin filaments elongate fromtheir membrane-associated ends. Journal of Cell Biology 90, 485–94.
TILNEY, L. G., & TILNEY, M. S. (1994) Methods to visualize actin polymerization associated with bacterial invasion. Methods in Enzymology 236, 476–81.
WANG, S.-M., CHEN, J.-S., FONG, T.-H., HSU, S.-Y. & LIM, S.-S. (1997) Characterization of a novel filament system in goldfish xanthophores. Cell Motility and the Cytoskeleton 36, 216–27.
YAMASHITA, R. A. & MAY, G. S. (1998) Motoring along the hyphae: molecular motors and the fungal cytoskeleton. Current Opinion in Cell Biology 10, 74–9.
ZACKROFF, R. V. & GOLDMAN, R. D. (1980) In vitro reassembly of squid brain intermediate filaments (neurofilaments): Purification by assembly-disassembly. Science 208, 1152–54.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bearer, E.L., Reese, T.S. Association of actin filaments with axonal microtubule tracts. J Neurocytol 28, 85–98 (1999). https://doi.org/10.1023/A:1007025421849
Issue Date:
DOI: https://doi.org/10.1023/A:1007025421849