Abstract
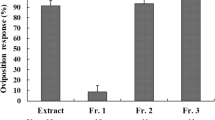
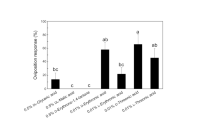
In nature, Papilio polytes utilizes a limited range of rutaceous plants as hosts. We isolated and identified oviposition stimulants for the butterfly from the foliage of its primary host plant Toddalia asiatica. Females readily deposited eggs in response to a methanolic extract of the plant. Partition of the extract with organic solvents revealed that chemicals responsible for eliciting egg-laying resided in a water-soluble fraction. Further bioassay-guided fractionation of the active fraction by column chromatography, preparative TLC, and HPLC led to the isolation of two oviposition stimulants. One was isolated from an amphoteric fraction and identified as trans-4-hydroxy-N-methyl-L-proline [(−)-(2S,4R)-4-hydroxy-1-methyl pyrrolidine-2-carboxylic acid; HMP]. The other, isolated from an acidic fraction, was identified as 2-C-methyl-D-erythronic acid [(−)-(2R,3R)-2-methyl-2,3,4-trihydroxybutanoic acid; MEA]. HMP alone evoked significant oviposition-stimulatory activity, although this was much lower than that of the original water-soluble fraction. MEA, on the other hand, alone did not elicit positive responses from females. However, HMP, when assayed in combination with MEA, markedly enhanced the female response, and the mixture was as active in stimulating oviposition as were the original water-soluble fraction and the plant foliage. We conclude that HMP is a substance crucial for host recognition by females, while MEA is a synergistic stimulant involved in host recognition and/or preference.
Similar content being viewed by others
References
Ahmed, A. A., Spring, O., El-Razek, M. H. A., Hussein, N. S., and Mabry, T. J. 1995. Sesquiterpene lactones and other constituents from Hymenoxys richarsonii and H. subintegra. Phytochemistry 39:1127–1131.
Aubert, J., Legal, L., Descimon, H., and Michel, F. 1999. Molecular phylogeny of swallowtail butterflies of the tribe Papilionini (Papilionidae, Lepidoptera). Mol. Phylogenet. Evol. 12:156–167.
Baur, R. and Feeny, P. 1995. Comparative electrophysiological analysis of plant odor perception in females of three Papilio species. Chemoecology 5/6:26–36.
Baur, R., Feeny, P., and Städler, E. 1993. Oviposition stimulants for the black swallowtail butterfly: Identification of electrophysiologically active compounds in carrot volatiles. J. Chem. Ecol. 19:919–937.
Bell, E. A. 1977. The possible significance of uncommon amino acids in plant-vertebrate, plant-insect and plant-plant relationships, pp. 571-595, in G. B. Marini-Bettolo (ed.). Natural Products and the Protection of Plants. Elsevier, Amsterdam, the Netherlands.
Bell, E. A. 1981. The physiological role(s) of secondary (natural) products, pp. 1-17, in E. E. Conn, (ed.). The Biochemistry of Plants: A Comprehensive Treatise, Vol. 7: Secondary Plant Products. Academic Press, New York.
Bernays, E. A. 1983. Nitrogen in defence against insects, pp. 321-344, in J. A. Lee, S. McNeil, and I. H. Rorison (Eds.). Nitrogen as an Ecological Factor. Blackwell Scientific, Oxford.
Brenner, S. A. and J. T. Romeo. 1986. Fungitoxic effects of nonprotein imino acids on the growth of saprophytic fungi isolated from the leaf surface of Calliandra haematocephala. Appl. Env. Microbiol. 51:690–693.
Carter, M., Feeny, P., and Haribal, M. 1999. An oviposition stimulant for spicebush swallowtail butterfly, Papilio troilus, from leaves of Sassafras albidum. J. Chem. Ecol. 25:1233–1245.
Carter, M., Sachdev-Gupta, K., and Feeny, P. 1998. Tyramine isolated from parsnip leaves: A stimulant and synergist for oviposition of the black swallowtail. Physiol. Entomol. 23:303–312.
Dreyer, D. L. 1966. Citrus bitter principles-V. Botanical distribution and chemotaxonomy in the Rutaceae. Phytochemistry 5:367–378.
Feeny, P. 1992. The evolution of chemical ecology: Contributions from the study of herbivorous insects, pp. 1-44, in G. A. Rosenthal and M. R. Berenbaum (Eds.). Herbivores: Their Interactions with Secondary Metabolites, Vol. II: Ecological and Evolutionary Processes. Academic Press, San Diego, California.
Feeny, P., Sachdev, K., Rosenberry, L., and Cater, M. 1988. Luteolin 7-O-(6′-O-malonyl)-β-d-glucoside and trans-chlorogenic acid: Oviposition stimulants for the black swallowtail butterfly. Phytochemistry 27:3439–3448.
Feeny, P., Städler, E., åhman, I., and Carter, M. 1989. Effect of plant odor on oviposition by the black swallowtail butterfly, Papilio polyxenes (Lepidoptera: Papilionidae). J. Insect Behav. 2:803–827.
Figliuolo, R., Naylor, S., Wang, J., and Langenheim, J. H. 1987. Unusual nonprotein imino acid and its relationship to phenolic and nitrogenous compounds in Copaifera. Phytochemistry 26:3255–3259.
Ford, C. W. 1981. A new lactone from water-stressed chickpea. Phytochemistry 20:2019–2020.
Goodson, J. A. and Clewer, H. W. B. 1919. Examination of the bark of Croton gubouga. Isolation of 4-hydroxyhygric acid. J. Chem. Soc. 923–933.
Haribal, M. and Feeny, P. 1998. Oviposition stimulant for the zebra swallowtail butterfly, Eurytides marcellus, from the foliage of pawpaw, Asimina triloba. Chemoecology 8:99–110.
Honda, K. 1986. Flavanone glycosides as oviposition stimulants in a papilionid butterfly, Papilio protenor. J. Chem. Ecol. 12:1999–2010.
Honda, K. 1990. Identification of host-plant chemicals stimulating oviposition by swallowtail butterfly, Papilio protenor. J. Chem. Ecol. 16:325–337.
Honda, K. 1995. Chemical basis of differential oviposition by lepidopterous insects. Arch. Insect Biochem. Physiol. 30:1–23.
Honda, K., Hayashi, N., Abe, F., and Yamauchi, T. 1997a. Pyrrolizidine alkaloids mediate host-plant recognition by ovipositing females of an Old World danaid butterfly, Idea leuconoe. J. Chem. Ecol. 23:1703–1713.
Honda, K., Kawano S., and Hayashi, N. 1997b. Flavonoids as oviposition stimulants or deterrents in host selection by swallowtail butterflies (Papilio), p. 259, in Abstracts of the Third Asia-Pacific Conference of Entomology (APCEIII).
Honda, K. and Nishida, R. 1999. Oviposition stimulants and deterrents in butterflies, pp. 330-350, in Honda, K., Honda, H., and Tatsuki, S. (Eds.). Environmental Entomology: Behavior, Physiology and Chemical Ecology. University of Tokyo Press, Tokyo (in Japanese).
Jones, G. P., Naidu, B. P., Paleg, L. G., Tiekink, E. R. T., and Snow, M. R. 1987. 4-Hydroxy-N-methylproline analogues in Melaleuca spp. Phytochemistry 26:3343–3344.
Miller, J. R. and Strickler, K. L. 1984. Finding and accepting host plants, pp. 127-157, in J. B., Willam, and R. T. Cardé (Eds.). Chemical Ecology of Insects. Chapman and Hall, New York.
Miller, J. S. 1987a. Phylogenetic studies in the Papilioninae (Lepidoptera: Papilionidae). Bull. Am. Mus. Nat. Hist. 186:385–512.
Miller, J. S. 1987b. Host-plant relationships in the Papilionidae (Lepidoptera): Parallel cladogenesis or colonization? Cladistics 3:105–120.
Morgan, J. W. W. 1964. 4-Hydroxy-N-methyl-l-proline from Afromosia elata heartwood. Chem. Ind. 28:542–543.
Murakami, T., Honda, K., Nakayama, T., and Hayashi, N. 2003. Phytochemical-mediated differential acceptance of four rutaceous plants by a swallowtail butterfly, Papilio polytes (Lepidoptera: Papilionidae). Appl. Entomol. Zool. 38:37–43.
Nakayama, T., Honda, K., and Hayashi, N. 2002. Chemical mediation of differential oviposition and larval survival on rutaceous plants in a swallowtail butterfly, Papilio polytes. Entomol. Exp. Appl. 105:35–42.
Nishida, R. 1995. Oviposition stimulants of swallowtail butterflies, pp. 17-26. in J. M. Scriber, Y. Tsubaki, and R. C. Lederhouse (Eds.). Swallowtail Butterflies: Their Ecology and Evolutionary Biology. Scientific Publishers, Gainesville, Florida.
Nishida, R. and Fukami, H. 1989. Oviposition stimulants of an Aristolochiaceae-feeding swallowtail butterfly, Atrophaneura alcinous. J. Chem. Ecol. 15:2565–2575.
Nishida, R., Ohsugi, T., Kokubo, S., and Fukami, H. 1987. Oviposition stimulants of a Citrus-feeding swallowtail butterfly, Papilio xuthus L. Experientia 43:342–344.
Ohsugi, T., Nishida, R., and Fukami, H. 1985. Oviposition stimulants of Papilio xuthus, a Citrus-feeding swallowtail butterfly. Agric. Biol. Chem. 49:1897–1900.
Ohsugi, T., Nishida, R., and Fukami, H. 1991. Multi-component system of oviposition stimulants for a Rutaceae-feeding swallowtail butterfly, Papilio xuthus (Lepidoptera: Papilionidae). Appl. Entomol. Zool. 26:29–40.
Ono, H., Nisihda, R., and Kuwahara, Y. 2000a. Oviposition stimulant for a Rutaceae-feeding swallowtail butterfly, Papilio bianor (Lepidoptera: Papilionidae): Hydroxycinnamic acid derivative from Orixa japonica. Appl. Entomol. Zool. 35:119–123.
Ono, H., Nishida, R., and Kuwahara, Y. 2000b. A dihydroxy-γ-lactone as an oviposition stimulant for the swallowtail butterfly, Papilio bianor, from the rutaceous plant, Orixa japonica. Biosci. Biotechnol. Biochem. 64:1970–1973.
Renwick, J.A.A. and Chew, F.S. 1994. Oviposition behavior in Lepidoptera. Annu. Rev. Entomol. 39:377–400.
Romeo, J. T. 1998. Functional multiplicity among nonprotein amino acids in mimosoid legumes: a case against redundance. Ecoscience 5:287–294.
Romeo, J. T. and M. S. J. Simmonds. 1989. Nonprotein amino acid feeding deterrents from Calliandra, pp. 59-68, in J. T. Arnason, B. J. R. Philogene, and P. Morland (Eds.). Pesticides of Plant Origin, ACS Symposium Series No. 387. American Chemical Society, Washington, DC.
Rosenthal, G. A. 1982. Plant Nonprotein Amino and Imino Acids: Biological, Biochemical and Toxicological Properties. Academic Press, New York.
Rosenthal, G. A. and Bell, E. A. 1979. Naturally occurring, toxic nonprotein amino acids, pp. 353-385, in G. A. Rosenthal and D. A. Janzen (Eds.). Herbivores: Their Interactions with Secondary Plant Metabolites. Academic Press, New York.
Sachdev-Gupta, K., Feeny, P. P., and Carter, M. 1993. Oviposition stimulants for the pipevine swallowtail butterfly, Battus philenor (Papilionidae), from an Aristolochia host plant: Synergism between inositols, aristolochic acids and a monogalactosyl diglyceride. Chemoecology 4:19–28.
Schramm, R. W., Tomaszewska, B., and Petersson, G. 1979. Sugar-related hydroxy acids from Phaseolus and Trifolium species. Phytochemistry 18:1393–1394.
Sciuto, S., Chillemi, R., Piattelli, M., and Impellizzeri, G. 1983. The identification of 4-hydroxy-N-methylproline in the red alga Chondria coerulescens-spectral information. Phytochemistry 22:2311–2312.
Sondengam, B. L., Hémo, J. H., and Charles, G. 1973. Sur une nouvelle methode de methylation des amines primaires et secondaires. Tetrahedron Lett. 14:261–263.
Städler, E. 1992. Behavioral responses of insects to plant secondary compounds, pp. 45-48, in G. A. Rosenthal and M. R. Berenbaum (Eds.). Ecological and Evolutionary Processes, Vol. II: Herbivores: Their Interactions with Secondary Metabolites. Academic Press, San Diego, California.
Teresa, J. P., Aubanell, J. C. H., Feliciano, A. S., and Corral J. M. M. 1980. Saccharinic acid lactone from Astragalus lusitanicus Lam. (–)-2-C-methyl-d-erythrono-1,4-lactone. Tetrahedron Lett. 21:1359–1360.
Tyler, H., Brown, K.S., and Wilson, K. 1994. Swallowtail Butterflies of the Americas. Scientific Publishers Gainesville, Florida.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakayama, T., Honda, K., Ômura, H. et al. Oviposition Stimulants for the Tropical Swallowtail Butterfly, Papilio polytes, Feeding on a Rutaceous Plant, Toddalia asiatica . J Chem Ecol 29, 1621–1634 (2003). https://doi.org/10.1023/A:1024274814402
Issue Date:
DOI: https://doi.org/10.1023/A:1024274814402