Abstract
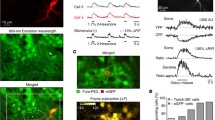
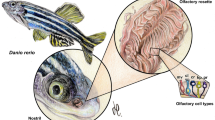
Olfactory coding at the level of the olfactory bulb is thought to depend upon an ensemble response of mitral cells receiving input from chemotopically-organized projections of olfactory sensory neurons and regulated by lateral inhibitory circuits. Immunocytochemical methods are described to metabolically classify neurons in the developing zebrafish olfactory system based on the relative concentrations of taurine, glutamate, GABA (and potentially other small biogenic amines) and a small guanidium-based cation, agmatine, which labels NMDA-sensitive cells by permeating through active ionotropic glutamate receptor channels. Using metabolic profiling in conjunction with activity dependent labeling we demonstrate that neuronal differentiation in the developing olfactory bulb, as assessed by acquisition of a mature neurochemical profile, and sensitivity to an ionotropic glutamate receptor agonist, NMDA, occurs during the second day of development. This experimental approach is likely to be useful in studies concerned with the development of glutamatergic signaling pathways.
Similar content being viewed by others
References
Buck LB (1996). Information coding in the vertebrate olfactory system. Annu Rev Neurosci 19: 517–544.
Buck L, Axel R (1991). A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell 65: 175–187.
Ressler KJ, Sullivan SL, Buck LB (1994). Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell 79: 1245–1255.
Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R (1996). Visualizing an olfactory sensory map. Cell 87: 675–686.
Mori K, Nagao H, Yoshihara Y (1999). The olfactory bulb: Coding and processing of odor molecule information. Science 286: 711–715.
Mori K, Nagao H, Sasaki YF (1998). Computation of molecular information in mammalian olfactory systems. Network 9: R79–102.
Mori K, Yoshihara Y (1995). Molecular recognition and olfactory processing in the mammalian olfactory system. Prog Neurobiol 45: 585–619.
Hara TJ (1994). Olfaction and gustation in fish: an overview. Acta Physiol Scand 152: 207–217.
Barth AL, Justice NJ, Ngai J (1996). Asynchronous onset of odorant receptor expression in the developing zebrafish olfactory system. Neuron 16: 23–34.
Friedrich RW, Korsching SI (1997). Combinatorial and chemotopic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron 18: 737–752.
Friedrich RW, Korsching SI (1998). Chemotopic, combinatorial, and noncombinatorial odorant representations in the olfactory bulb revealed using a voltage-sensitive axon tracer. J Neurosci 18: 9977–9988.
Fuss SH, Korsching SI (2001). Odorant feature detection: activity mapping of structure response relationships in the zebrafish olfactory bulb. J Neurosci 21: 8396–8407.
Edwards JG, Michel WC (2002). Odor-stimulated glutamatergic neurotransmission in the zebrafish olfactory bulb. J Comp Neurol 454: 294–309.
Friedrich RW, Laurent G (2001). Dynamic optimization of odor representations by slow temporal patterning of mitral cell activity. Science 291: 889–894.
Whitlock KE, Westerfield M (2000). The olfactory placodes of the zebrafish form by convergence of cellular fields at the edge of the neural plate. Development 127: 3645–3653.
Hansen A, Zeiske E (1993). Development of the olfactory organ in the zebrafish, Brachydanio rerio. J Comp Neurol 333: 289–300.
Whitlock KE, Westerfield M (1998). A transient population of neurons pioneers the olfactory pathway in the zebrafish. J Neurosci 18: 8919–8927.
Dynes JL and Ngai J (1998). Pathfinding of olfactory neuron axons to stereotyped glomerular targets revealed by dynamic imaging in living zebrafish embryos. Neuron 20: 1081–1091.
Marc RE, Cameron D (2001). A molecular phenotype atlas of the zebrafish retina. J Neurocytol 30: 593–654.
Marc RE, Murry RF, Fisher SK, Linberg KA, Lewis GP, Kalloniatis M (1998). Amino acid signatures in the normal cat retina. Invest Ophthalmol Vis Sci 39: 1685–1693.
Kalloniatis M, Marc RE, Murry RF (1996). Amino acid signatures in the primate retina. J Neurosci 16: 6807–6829.
Yoshikami D (1981). Transmitter sensitivity of neurons assayed by autoradiography. Science 212: 929–930.
Marc RE (1999). Mapping glutamatergic drive in the vertebrate retina with a channel permeant organic cation. J Comp Neurol 407: 47–64.
Marc RE (1999). Kainate activation of horizontal, bipolar, amacrine and ganglion cells in the rabbit retina. J Comp Neurol 407: 65–76.
Raasch W, Regunathan S, Li G, Reis DJ (1995). Agmatine, the bacterial amine, is widely distributed in mammalian tissues. Life Sciences 56: 2319–2330.
Reis DJ and Regunathan S (1999). Agmatine: an endogenous ligand at imidazoline receptors is a novel neurotransmitter [In Process Citation]. Ann N Y Acad Sci 881: 65–80.
Westerfield M (1995). The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio). 3rd ed.
Marc RE, Murry RF, Basinger SF (1995). Pattern recognition of amino acid signatures in retinal neurons. J Neurosci 15: 5106–5129.
Lipschitz DL, Michel WC (2002). Amino acid odorants stimulate microvillar sensory neurons. Chem Senses 27: 277–286.
Lipschitz DL, Michel WC (1999). Guanidium analogs are detected by multiple odorant receptors in the zebrafish (Danio rerio) olfactory system. Chem Senses 24: 593.
Zou Z, Horowitz LF, Montmayeur JP, Snapper S, Buck LB (2001). Genetic tracing reveals a stereotyped sensory map in the olfactory cortex. Nature 414: 173–179.
Celik A, Fuss SH, Korsching SI (2002). Selective targeting of zebrafish olfactory receptor neurons by the endogenous OMP promoter. Eur J Neurosci 15: 798–806.
Yoshida T, Ito A, Matsuda N, Mishina M (2002). Regulation by protein kinase A switching of axonal pathfinding of zebrafish olfactory sensory neurons through the olfactory placode-olfactory bulb boundary. J Neurosci 22: 4964–4972.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sakata, Y., Olson, J.K. & Michel, W.C. Assessment of neuronal maturation and acquisition of functional competence in the developing zebrafish olfactory system. Methods Cell Sci 25, 39–48 (2003). https://doi.org/10.1023/B:MICS.0000006852.16798.34
Issue Date:
DOI: https://doi.org/10.1023/B:MICS.0000006852.16798.34