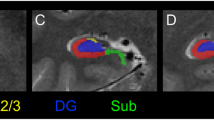
Abstract
Poor attention and impaired memory are enduring and core features of schizophrenia. These impairments have been attributed either to global cortical dysfunction or to perturbations of specific components associated with the dorsolateral prefrontal cortex (DLPFC), hippocampus and cerebellum. Here, we used positron emission tomography (PET) to dissociate activations in DLPFC and hippocampus during verbal episodic memory retrieval. We found reduced hippocampal activation during conscious recollection of studied words, but robust activation of the DLPFC during the effort to retrieve poorly encoded material in schizophrenic patients. This finding provides the first evidence of hippocampal dysfunction during episodic memory retrieval in schizophrenia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kraepelin, E. Dementia Praecox and Paraphrenia (Livingstone, Edinburgh, 1919).
Goldberg, T.E. & Gold, J.M. in Schizophrenia (eds Hirsch, S. R. & Weinberger, D. R.) 146–162 (Blackwell Science Ltd, Oxford, 1995).
Green, M.F. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry 153, 321–330 (1996).
Andreasen, N.C. Pieces of the schizophrenia puzzle fall into place. Neuron 16 , 697–700 (1996).
Andreasen, N.C. Linking mind and brain in the study of mental illnesses: a project for a scientific psychopathology. Science 275, 1586– 1593 (1997).
Berman, K.F., Zec, R.F. & Weinberger, D.R. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. II. Role of neuroleptic treatment, attention, and mental effort. Arch. Gen. Psychiatry 43, 126–135 (1986).
Andreasen, N.C. et al. Schizophrenia and cognitive dysmetria: A positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc. Natl. Acad. Sci. USA 93, 9985–9990 (1996).
Liddle, P.F. et al. Patterns of cerebral blood flow in schizophrenia. Br. J. Psychiatry 160, 179–186 ( 1992).
Silbersweig, D.A. et al. A functional neuroanatomy of hallucinations in schizophrenia. Nature 378, 176–179 ( 1995).
Ungerleider, L.G. Functional brain imaging studies of cortical mechanisms for memory. Science 270, 769–775 ( 1995).
Fletcher, P.C., Frith, C.D. & Rugg, M.D. The functional neuroanatomy of episodic memory. Trends Neurosci. 20, 213–218 (1997).
Goldman-Rakic, P.S. & Selemon, L.D. Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophrenia Bull. 23, 437–458 ( 1997).
Arnold, S.E. The medial temporal lobe in schizophrenia. J. Neuropsychiatry Clin. Neurosci. 9, 460–470 ( 1997).
Schacter, D.L., Alpert, N.M., Savage, C.R., Rauch, S.L. & Albert, M.S. Conscious recollection and the human hippocampal formation: evidence from positron emission tomography. Proc. Natl. Acad. Sci. USA 93, 321– 325 (1996).
Dolan, R.J. & Fletcher, P.C. Dissociating prefrontal and hippocampal function in episodic memory encoding. Nature 388, 582–585 (1997).
Henke, K., Buck, A., Weber, B. & Wieser, H.G. Human hippocampus establishes associations in memory. Hippocampus 7, 249– 256 (1997).
Schacter, D.L., Savage, C.R., Alpert, N.M., Rauch, S.L. & Albert, M.S. The role of hippocampus and frontal cortex in age-related memory changes: a PET study. Neuroreport 7, 1165–1169 ( 1996).
Nyberg, L., McIntosh, A.R., Houle, S., Nilsson, L.-G. & Tulving, E. Activation of medial temporal structures during episodic memory retrieval. Nature 380, 715– 717 (1996).
Squire, L.R. et al. Activation of the hippocampus in normal humans: a functional anatomical study of memory. Proc. Natl. Acad. Sci. USA 89, 1837–1841 (1992).
Tulving, E., Kapur, S., Craik, F.I.M., Moscovitch, M. & Houle, S. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc. Natl. Acad. Sci. USA 91, 2016–2020 ( 1994).
Buckner, R.L. et al. Functional anatomical studies of explicit and implicit memory retrieval tasks . J. Neurosci. 15, 12–29 (1995).
Schacter, D.L. et al. Neuroanatomical correlates of veridical and illusory recognition memory: evidence from positron emission tomography. Neuron 17, 267–274 (1996).
Spitzer, M., Braun, U., Hermle, L. & Maier, S. Associative semantic network dysfunction in thought-disordered schizophrenic patients: direct evidence from indirect semantic priming. Biol. Psychiatry 34 , 864–877 (1993).
Huron, C. et al. Impairment of recognition memory with, but not without, conscious recollection in schizophrenia. Am. J. Psychiatry 152, 1737–1742 (1995).
Venables, P.H. Hippocampal function and schizophrenia. Experimental psychological evidence. Ann. NY Acad. Sci. 658, 111–127 (1992).
Krieckhaus, E.E., Donahoe, J.W. & Morgan, M.A. Paranoid schizophrenia may be caused by dopamine hyperactivity of CA1 hippocampus. Biol. Psychiatry 31, 560–570 (1992).
Dwork, A.J. Postmortem studies of the hippocampal formation in schizophrenia. Schizophrenia Bull. 23, 403–421 ( 1997).
Beauregard, M. & Bachevalier, J. Neonatal insult to the hippocampal region and schizophrenia: a review and a putative animal model. Can. J. Psychiatry 41, 446–456 (1996).
Weinberger, D.R., Berman, K.F., Suddath, R. & Torrey, E.F. Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monzygotic twins. Am. J. Psychiatry 149, 890–897 (1992).
DeLisi, L.E. et al. Increased temporal lobe glucose use in chronic schizophrenic patients. Biol. Psychiatry 25, 835–851 (1989).
Friston, K.J., Liddle, P.F., Frith, C.D., Hirsch, S.R. & Frackowiak, R.S. The left medial temporal region and schizophrenia. A PET study. Brain 115, 367– 382 (1992).
Kawasaki, Y. et al. Regional cerebral blood flow in patients with schizophrenia: relevance to symptom structures. Psychiatry Res. 67, 49–58 (1996).
Frith, C.D. et al. Regional brain activity in chronic schizophrenic patients during the performance of a verbal fluency task. Br. J. Psychiatry 167, 343–349 (1995).
Goldman-Rakic, P.S. Working memory dysfunction in schizophrenia. J. Neuropsychiatry Clin. Neurosci. 6, 348–357 ( 1994).
Heaton, R. et al. Neuropsychological deficits in schizophrenics. Relationship to age, chronicity, and dementia. Arch. Gen. Psychiatry 51, 469–476 (1994).
Goldberg, T.E. & Weinberger, D.R. Effects of neuroleptic medication on the cognition of patients with schizophrenia: a review of recent studies . J. Clin. Psychiatry 57 [suppl 9], 62– 65 (1996).
Miller, D.D., Rezai, K., Alliger, R. & Andreasen, N.C. The effect of antipsychotic medication on relative cerebral blood perfusion in schizophrenia: assessment with technetium-99m hexamethyl- propyleneamine oxime single photon emission computed tomography. Biol. Psychiatry 41, 550–559 (1997).
Gilbertson, M.W. & van Kammen, D.P. Recent and remote memory dissociation: medication effects and hippocampal function in schizophrenia. Biol. Psychiatry 42, 585–595 (1997).
Tamminga, C.A. et al. Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch. Gen. Psychiatry 49, 522– 530 (1992).
Spitzer, R.L., Williams, J.B.W., Gibbon, M. & First, M.B. Structured clinical interview for DSM-III-R. (American Psychiatric Press, Washington, DC, 1991).
Diagnostic and Statistical Manual of Mental Disorders, 4th edition . 273–290 (American Psychiatric Association, Washington, DC, 1994).
Kay, S.R., Fiszbein, A. & Opler, L.A. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bull. 13, 261–276 (1987).
Alpert, N.M., Berdichevsky, D., Levin, Z., Morris, E.D. & Fishman, A.J. Improved methods for image registration. Neuroimage 3, 10–18 ( 1996).
Woods, R.P. Modeling for intergroup comparisons of imaging data. Neuroimage 4, S84–S94 (1996).
Acknowledgements
The authors thank Dmitry Berdichevsky, Zakhar Levin, Avis Loring, Steve Weise, Ed Amico and Dana Ruther for technical support. This study was supported by a Dupont-Warren Fellowship (S.H.), a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression (S.L.R.) and NIMH grants R01MH57915 (D.L.S.) and MH01215 (S.L.R.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heckers, S., Rauch, S., Goff, D. et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci 1, 318–323 (1998). https://doi.org/10.1038/1137
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/1137
This article is cited by
-
Confidence in visual detection, familiarity and recollection judgments is preserved in schizophrenia spectrum disorder
Schizophrenia (2023)
-
Glutamatergic dysfunction leads to a hyper-dopaminergic phenotype through deficits in short-term habituation: a mechanism for aberrant salience
Molecular Psychiatry (2023)
-
Discrete hippocampal projections are differentially regulated by parvalbumin and somatostatin interneurons
Nature Communications (2023)
-
Relevance of interactions between dopamine and glutamate neurotransmission in schizophrenia
Molecular Psychiatry (2022)
-
Multilevel segmentation of Hippocampus images using global steered quantum inspired firefly algorithm
Applied Intelligence (2022)