Abstract
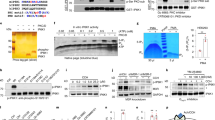
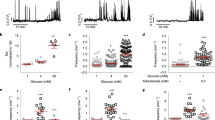
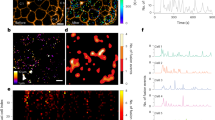
Insulin is produced from an inactive precursor, proinsulin, through initial endoproteolytic cleavage at sites marked by pairs of basic amino-acid residues1,2. We report here that lysates of insulin secretory granules contain two distinct Ca-dependent acidic endoproteases; one (type I) cleaving exclusively on the C-terminal side of Arg 31.Arg 32 (B-chain/C-peptide junction), the other (type II) preferentially on the C-terminal side of Lys 64.Arg 65 of proinsulin (C-peptide/A-chain junction). The Ca and pH requirements of these proteinases suggested that the type-II pro-teinase would be active in the Golgi apparatus and the secretory granule, whereas type-I activity would be compatible only with the intragranular environment. Kinetic analyses of (pro)insulin conversion intermediates in [35S]methionine-pulsed rat islets support this supposition. Our results suggest a simple mechanism whereby different dibasic sites can be cleaved in different cellular compartments. In conjunction with the regulation of the ionic composition of such compartments and the operation of post-Golgi segregation, our results also suggest how proteolytic conversion of diverse proproteins destined for different cellular sites can occur differentially and in a regulated manner.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Docherty, K. D. & Steiner, D. F. A. Rev. Physiol. 44, 625–638 (1982).
Loh, Y. P., Brownstein, M. J. & Gainer, H. A. Rev. Neurosci. 7, 189–222 (1984).
Davidson, H. W., Peshavaria, M. & Hutton, J. C. Biochem. J. 246, 279–286 (1987).
Hutton, J. C., Davidson, H. W. & Peshavaria, M. Biochem. J. 244, 457–464 (1987).
Hutton, J. C. Biochem. J. 204, 171–178 (1982).
Hutton, J. C., Penn, E. J. & Peshavaria, M. Biochem. J. 210, 297–305 (1983).
Orci, L. et al. Cell 49, 865–868 (1987).
Herman, L., Sato, T. & Hales, C. N. J. Ultrastruct. Res. 42, 298–311 (1973).
Davidson, H. W. & Hutton, J. C. Biochem. J. 245, 575–582 (1987).
Given, B. D. et al. J. clin. Invest. 76, 1398–1405 (1985).
Orci, L. Diabetologia 28, 528–546 (1985).
Hutton, J. C. & Peshavaria, M. Biochem. J. 204, 161–170 (1982).
Rhodes, C. J., Lucas, C. A., Mutkoski, R. L., Orci, L. & Halban, P. A. J. biol. Chem. 262, 10712–10717 (1987).
Formby, B., Capito, K., Egeberg, J. & Hedeskov, C. J. Am. J. Physiol 230, 441–448 (1976).
Howell, S. L. & Tyhurst, M. J. Cell Sci. 21, 415–422 (1976).
Grant, P. T., Coombs, T. L. & Frank, B. H. Biochem. J. 126, 433–440 (1972).
Kohnert, K-D., Hahn, H-J., Gylfe, E., Borg, H. & Hellman, B. Molec. cell. Endocrinol. 16, 205–220 (1979).
Hutton, J. C., Davidson, H. W., Grimaldi, K. A. & Peshavaria, M. Biochem. J. 244, 449–456 (1987).
Grimaldi, K. A., Siddle, K. & Hutton, J. C. Biochem. J. 245, 567–573 (1987).
Howell, S. L., Tyhurst, M., Duvefelt, H., Andersson, A. & Hellerström, C. Cell Tissue Res. 188, 107–118 (1978).
Nagamatsu, S., Bolaffi, J. L. & Grodsky, G. M. Endocrinology 120, 1225–1231 (1987).
Rhodes, C. J. & Halban, P. A. J. Cell Biol. 105, 145–153 (1987).
Steiner, D. F. et al. Proc. natn. Acad. Sci. U.S.A. 84, 6184–6188 (1987).
Moore, H-P., Walker, M. D., Lee, F. & Kelly, R. B. Cell 35, 531–538 (1983).
Thim, L. et al. Proc. natn. Acad. Sci. U.S.A. 83, 6766–6770 (1986).
Brennan, S. O. & Peach, R. J. FEBS Lett. 229, 167–190 (1988).
Storer, A. C. & Cornish-Bowden, A. Biochem. J. 159, 1–5 (1976).
Lacy, P. E. & Kostianovsky, M. Diabetes 16, 35–39 (1967).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Davidson, H., Rhodes, C. & Hutton, J. Intraorganellar calcium and pH control proinsulin cleavage in the pancreatic β cell via two distinct site-specific endopeptidases. Nature 333, 93–96 (1988). https://doi.org/10.1038/333093a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/333093a0
This article is cited by
-
The Ykt6–Snap29–Syx13 SNARE complex promotes crinophagy via secretory granule fusion with Lamp1 carrier vesicles
Scientific Reports (2024)
-
Secretory granule exocytosis and its amplification by cAMP in pancreatic β-cells
Diabetology International (2022)
-
Insulin granule biogenesis and exocytosis
Cellular and Molecular Life Sciences (2021)
-
SLC30A family expression in the pancreatic islets of humans and mice: cellular localization in the β-cells
Journal of Molecular Histology (2018)
-
Loss of mTORC1 signalling impairs β-cell homeostasis and insulin processing
Nature Communications (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.