Abstract
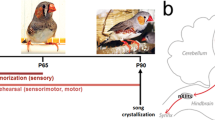
Birdsong, like human speech, is learned via auditory experience during a developmentally restricted sensitive period. Within projection neurons of two avian forebrain nuclei, NMDA receptor-mediated EPSCs (NMDA-EPSCs) become fast during song development, a transition posited to limit learning. To discover whether slow NMDA-EPSCs at these synapses are required for learning, we delayed song learning beyond its normal endpoint, post-hatch day (PHD) 65, by raising zebra finches in isolation from song tutors. At PHD45, before learning, isolation delayed NMDA-EPSC maturation, but only transiently. By PHD65, NMDA-EPSCs in isolates were fast and adult-like, yet isolates presented with tutors readily learned song. Thus song learning did not require slow NMDA-EPSCs at synapses critical for song development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Notes
Note: audio files of example songs from Fig. 2a can be found on the Nature Neuroscience web site ( http://www.nature.com/neuro/web_specials/).
References
Carmignoto, G. & Vicini, S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science 258, 1007–1011 ( 1992).
Hestrin, S. Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature 357, 686– 689 (1992).
Crair, M. R. & Malenka, R. C. A critical period for long-term potentiation at thalamocortical synapses. Nature 375 , 325–328 (1995).
Shi, J., Aamodt, S. M. & Constantine-Paton, M. Temporal correlations between functional and molecular changes in NMDA receptors and GABA neurotransmission in the superior colliculus. J. Neurosci. 17, 6264 –6276 (1997).
Livingston, F. S. & Mooney, R. Development of intrinsic and synaptic properties in a forebrain nucleus essential to avian song learning. J. Neurosci. 17, 8997– 9009 (1997).
White, S. A., Livingston, F. S. & Mooney, R. D. Androgens modulate NMDA receptor-mediated EPSCs in the zebra finch song system. J. Neurophysiol. 82, 2221–2234 (1999).
Tang, Y. et al. Genetic enhancement of learning and memory in mice. Nature 401, 63–69 ( 1999).
Korsia, S. & Bottjer, S. W. Chronic testosterone treatment impairs vocal learning in male zebra finches during a restricted period of development. J. Neurosci. 11, 2362– 2371 (1991).
Immelmann, K. in Bird Vocalisations (ed. Hinde, R. A.) 61–74 (Cambridge Univ. Press, London, 1969).
Eales, L. A. Song learning in zebra finches: some effects of song model availability on what is learnt and when. Anim. Behav. 33, 1293–1300 (1985).
Morrison, R. G. & Nottebohm, F. Role of a telencephalic nucleus in the delayed song learning of socially isolated zebra finches. J. Neurobiol. 24, 1045–1064 (1993).
Aamodt, S. M., Nordeen, E. J. & Nordeen, K. W. Early isolation from conspecific song does not affect the normal developmental decline of N -methyl-d-aspartate receptor binding in an avian song nucleus. J. Neurobiol. 27, 76–84 (1995).
Jones, A. E., ten Cate, C. & Slater, P. J. B. Early experience and plasticity of song in adult male zebra finches (Taeniopygia guttata). J. Comp. Psychol. 110, 354–369 (1996).
Nottebohm, F., Kelley, D. B. & Paton, J. A. Connections of vocal control nuclei in the canary telencephalon. J. Comp. Neurol. 207, 344 –357 (1982).
Mooney, R. Synaptic basis for developmental plasticity in a birdsong nucleus. J. Neurosci. 12, 2464–2477 (1992).
Bottjer, S. W., Miesner, E. A. & Arnold, A. P. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science 224, 901–903 (1984).
Scharff, C. & Nottebohm, F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J. Neurosci. 11, 2896–2913 (1991).
Nottebohm, F., Stokes, T. M. & Leonard, C. M. Central control of song in the canary, Serinus canarius. J. Comp. Neurol. 165, 457– 486 (1976).
McCasland, J. S. Neuronal control of birdsong production. J. Neurosci. 7, 23–39 (1987).
Yu, A. C., Dave, A. S. & Margoliash, D. Temporal hierarchical control of singing in birds. Science 273, 1871–1875 ( 1996).
Basham, M. E., Nordeen, E. J. & Nordeen, K. M. Blockade of NMDA receptors in the anterior forebrain impairs sensory acquisition in the zebra finch (Poephila guttata). Neurobiol. Learn. Mem. 66, 295–304 (1996).
Konishi, M. The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Z. Tierpsychol. 22, 770– 783 (1965).
Price, P. H. Developmental determinants of structure in zebra finch song. J. Comp. Physiol. Psychol. 93, 260–277 (1979).
Arnold, A. P. The effects of castration on song development in zebra finches (Poephila guttata). J. Exp. Zool. 191, 261– 278 (1975).
Bottjer, S. W. & Hewer, S. J. Castration and antisteroid treatment impair vocal learning in male zebra finches. J. Neurobiol. 23, 337–353 (1992).
Prove, E. in Hormones and Behavior in Higher Vertebrates (eds. Balthazart, J., Prove, E. & Gilles, R.) 368–374 (Springer, Berlin, 1983).
Wingfield, J. C. & Moore, M. C. in Psychobiology of Reproduction: An Evolutionary Approach (ed. Crews, D.) 149– 175 (Prentice Hall, Englewood Cliffs, New Jersey, 1987 ).
Doupe, A. J. Song- and order-selective neurons in the songbird anterior forebrain and their emergence during vocal development. J. Neurosci. 17 , 1147–1167 (1997).
Marler, P., Peters, S., Ball, G. F., Dufty, A. M. Jr. & Wingfield, J. C. The role of sex steroids in the acquisition and production of birdsong. Nature 336, 770–772 (1988).
Hubel, D. H., Wiesel, T. N. & LeVay, S. Plasticity of ocular dominance columns in monkey striate cortex. Phil. Trans. R. Soc. Lond. B Biol. Sci. 278 , 377–409 (1977).
Quinlan, E. M., Philpot, B. D., Huganir, R. L. & Bear, M. F. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat. Neurosci. 2, 352–357 (1999).
Flint, A. C., Maisch, U. S., Weishaupt, J. H., Kriegstein, A. R. & Monyer, H. NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J. Neurosci. 17, 2469–2476 (1997).
Roberts, E. B. & Ramoa, A. S. Enhanced NR2A subunit expression and decreased NMDA receptor decay time at the onset of ocular dominance plasticity in the ferret. J. Neurophysiol. 81, 2587–2591 (1999).
Wallhausser-Franke, E., Nixdorf-Bergweiler, B. E. & DeVoogd, T. J. Song isolation is associated with maintaining high spine frequencies on zebra finch LMAN neurons. Neurobiol. Learn. Mem. 64, 25–35 ( 1995).
Stark, L. L. & Perkel, D. J. Two-stage, input-specific synaptic maturation in a nucleus essential for vocal production in the zebra finch . J. Neurosci. 19, 9107– 9116 (1999)
Nordeen, K. W. & Nordeen, E. J. Auditory feedback is necessary for the maintenance of stereotyped song in adult zebra finches . Behav. Neural Biol. 57, 58– 66 (1992).
Mayer, M. L., Westbrook, G. L. & Guthrie, P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature 309, 261–263 (1984).
Nowak, L., Bregestovski, P., Ascher, P., Herbet, A. & Prochiantz, A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature 307, 462–465 (1984).
Acknowledgements
We thank Dona Chikaraishi, Mike Ehlers, Felix Schweizer and all members of the Mooney lab for providing discussion of the manuscript. In particular, J. Matthew Kittelberger assisted with song analysis and Stacey S. James designed and constructed vinyl covers for isolation cages. In addition, Eugene A. Zimmerman gave assistance with the hormone measurements, and Mark Schmidt taught us the deafening technique. This research was supported by NRSA F31 MH11872 to F.S.L., H.H. Whitney fellowship to S.A.W. and by NIH R01 DC02524, McKnight, Klingenstein and Sloan Foundation awards to R.M.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Requires quicktime plug-in, download quicktime now
Spectrograms for one isolate, one control and one tutor. Click on a spectrogram to listen to the song.





Rights and permissions
About this article
Cite this article
Livingston, F., White, S. & Mooney, R. Slow NMDA-EPSCs at synapses critical for song development are not required for song learning in zebra finches. Nat Neurosci 3, 482–488 (2000). https://doi.org/10.1038/74857
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/74857
This article is cited by
-
Tracing development of song memory with fMRI in zebra finches after a second tutoring experience
Communications Biology (2023)
-
Vocal learning promotes patterned inhibitory connectivity
Nature Communications (2017)
-
Overexpression of human NR2B receptor subunit in LMAN causes stuttering and song sequence changes in adult zebra finches
Scientific Reports (2017)
-
Forebrain steroid levels fluctuate rapidly during social interactions
Nature Neuroscience (2008)
-
Neural mechanisms of birdsong memory
Nature Reviews Neuroscience (2006)